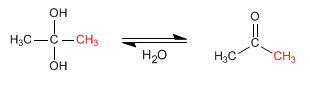Carboxylic acids react with two equivalents of organolytic followed by aqueous hydrolysis to form ketones.
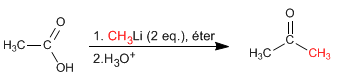
The reaction requires two equivalents of organolytic, the first deprotonates the acid group, while the second equivalent adds as a nucleophile to the carboxylic group.
The mechanism of the reaction occurs with the following stages:
Stage 1 . deprotonation of carboxylic acid
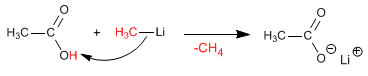
Stage 2 . Nucleophilic attack of the organometallic to the carboxylic group
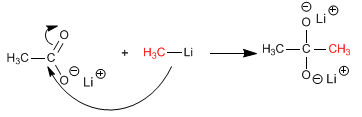
Stage 3. Formation of the hydrate by hydrolysis of the salt
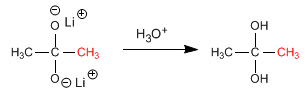
Stage 4. Conversion of the hydrate to ketone by loss of water.