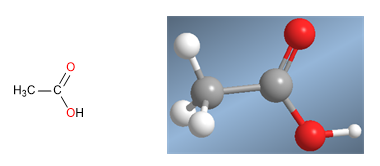
Carboxylic acids are molecules with trigonal planar geometry. They have acidic hydrogen in the hydroxyl group and behave like bases on carbonyl oxygen.
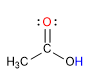
O : basic oxygen
H : Acidic hydrogen
The melting and boiling points are high since they form dimers, due to hydrogen bonding.
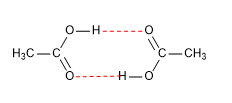
Hydrogen bonds ( ------------ )
CH3COOH Boiling point: 118ºC
CH3CH2COOH Boiling point: 141.8ºC
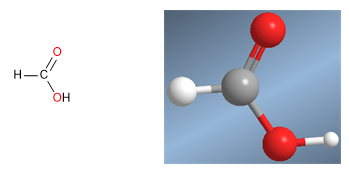
Methanoic acid molecular model
Ethanoic acid molecular model