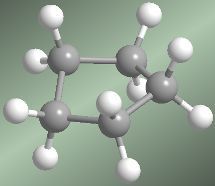
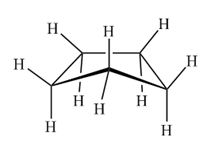
If cyclopentane were planar, it would be practically free of angle strain. But it would present five eclipsed hydrogens for each face, which would destabilize the molecule. The most favorable arrangement of cyclopentane is the envelope form. In this arrangement the sum of angular stresses and eclipsing is minimal.
 |
|