Cycles of six are the most abundant in nature. There are many compounds with biological activity whose base are cycles of six condensed atoms (cholesterol).
Cycles of six are the most abundant in nature. There are many compounds with biological activity whose base are cycles of six condensed atoms (cholesterol).
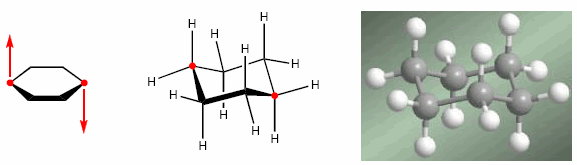
The explanation for this abundance lies in its stability. Cyclohexanes adopt a chair-shaped spatial arrangement, which allows them to minimize both angular strain and eclipsations. In the form of a chair, the bond angles are 111º, very close to 109.5º. In addition, all the hydrogens are alternated with respect to each other, minimizing the repulsions between them.