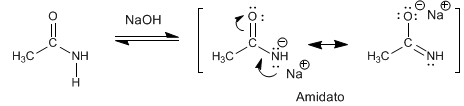
The amides have acidic hydrogens of Pka = 15 on the nitrogen atom. Deprotonation of the amino generates a resonance stabilized base, called amidate.
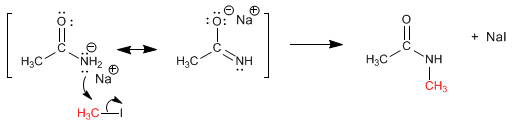
Amidates are nucleophilic species that usually act through nitrogen, attacking different electrophiles. Let's see the reaction between the obtained amidate and methyl iodide.