Draw the structures of the following ethers:
| 1) Butyl ethyl ether 2) Ethyl phenyl ether 3) Diphenyl ether 4) Divinyl ether 5) Isopropoxybutane 6) Benzyl phenyl ether 7) Methoxycyclohexane 8) 4-Methoxypent-2-ene 9) 4-ethoxybut-1-yne 10) Cyclohexyl phenyl ether | 11) 2-Chlorophenyl phenyl ether 12) tert-butyl isopropyl ether 13) 2-Methoxy-3-phenylbutan-1-ol 14) Diethyl ether 15) m-Ethoxyphenol 16) 2,3-Dimethyloxacyclopropane 17) 3-Methoxyoxacyclohexane 18) 2-Ethyl-3-methyloxacyclopentane 19) Cyclohexyl cyclopropyl ether 20) 2-Methoxypentane |
SOLUTION:
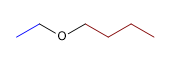
1) Butyl ethyl ether
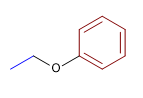
2) Ethyl phenyl ether
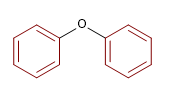
3) Diphenyl ether
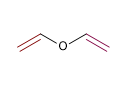
4) Divinyl ether
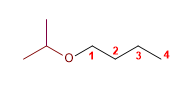
5) Isopropoxybutane
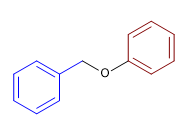
6) Benzyl phenyl ether
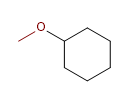
7) Methoxycyclohexane
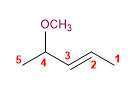
8) 4-Methoxypent-2-ene
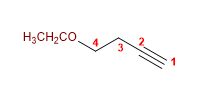
9) 4-ethoxybut-1-yne
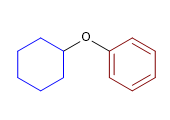
10) Cyclohexyl phenyl ether
![]() The alkoxide groups (methoxide, ethoxide...) are ordered alphabetically with the other substituents of the molecule and do not have any preference over them
The alkoxide groups (methoxide, ethoxide...) are ordered alphabetically with the other substituents of the molecule and do not have any preference over them
.
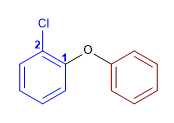
11) 2-Chlorophenyl phenyl ether
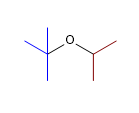
12) tert-butyl isopropyl ether
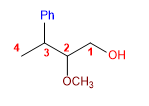
13) 2-Methoxy-3-phenylbutan-1-ol
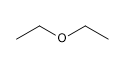
14) Diethyl ether
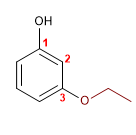
15) m-Ethoxyphenol
16) 2,3-Dimethyloxacyclopropane
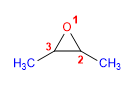
17) 3-Methoxyoxacyclohexane
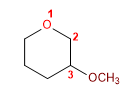
18) 2-Ethyl-3-methyloxacyclopentane
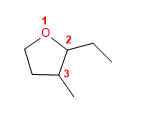
19) Cyclohexyl cyclopropyl ether
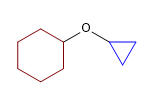
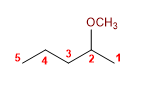
20) 2-Methoxypentane