Write the structural formula for each of the following alkanes.
| 1) 6-Isopropyl-2,5-dimethylnonane 2) 4-tert-butyl-3-methylheptane 3) Pentacosane 4) 4-Ethyl-4-methylheptane 5) 2,3-Dimethylpentane |
6) 5,5-Diethyl-2-methyl-4-propyldecane 7) 2,3,4-Trimethyloctane. 8) 4-tert-Butyloctane 9) 3-Ethyl-6,7-dimethyl-4-propyldodecane 10) 4,5-Diethyl-5-isopropyl-3,4-dimethyl-6-propylundecane |
SOLUTION:
1) 6-Isopropyl-2,5-dimethylnonane
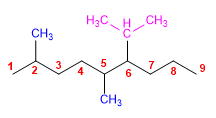
2) 4-tert-butyl-3-methylheptane
3) Pentacosane
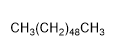
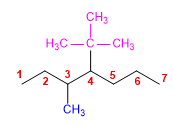
4) 4-Ethyl-4-methylheptane
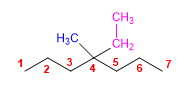
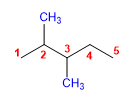
5) 2,3-Dimethylpentane
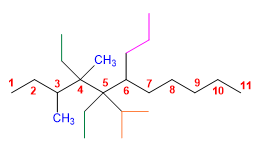
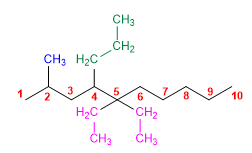
6) 5,5-Diethyl-2-methyl-4-propyldecane
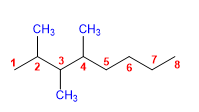
7) 2,3,4-Trimethyloctane.
8) 4-tert-Butyloctane
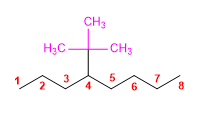
9) 3-Ethyl-6,7-dimethyl-4-propyldodecane
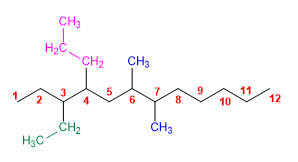
10) 4,5-Diethyl-5-isopropyl-3,4-dimethyl-6-propylundecane