Draw the most stable and least stable conformations of 2,3-dimethylpentane between carbons C3-C4.
SOLUTION:
The most stable conformation of the molecule is an alternate one. We draw the three alternating conformations and look for the one with the fewest gauche interactions.
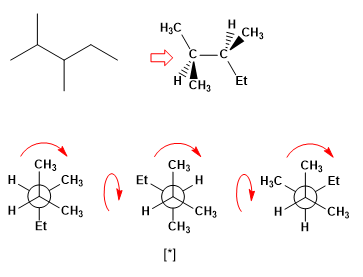
[*] More stable alternating conformation
The most unstable conformation of the molecule is an eclipsed one. We draw the three eclipsed conformations and find the one with the most eclipses.
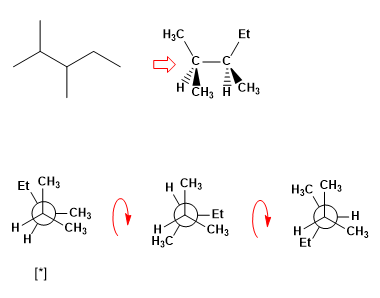
[*] More stable eclipsed conformation.