Which of the following compounds can exist in different conformations?
a) Hydrogen peroxide.
b) Ammonia.
c) Methanol.
SOLUTION:
For a compound to exist in different conformations, at least three consecutive single bonds are necessary. 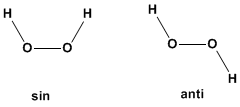 a) Hydrogen peroxide has infinite conformations that are obtained by rotation around the oxygen-oxygen bond. The conformation that has the two hydrogens facing each other is called SIN, the one with the hydrogens on opposite sides ANTI.
a) Hydrogen peroxide has infinite conformations that are obtained by rotation around the oxygen-oxygen bond. The conformation that has the two hydrogens facing each other is called SIN, the one with the hydrogens on opposite sides ANTI. 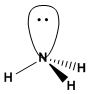 (b) Ammonia cannot exist in more than one conformation since it does not have consecutive bonds. The only bonds that can rotate are the nitrogen-hydrogen bonds and they do not produce any conformational change in the molecule.
(b) Ammonia cannot exist in more than one conformation since it does not have consecutive bonds. The only bonds that can rotate are the nitrogen-hydrogen bonds and they do not produce any conformational change in the molecule. 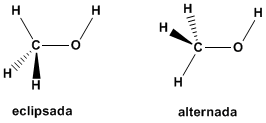 c) For methanol, two conformations can be drawn, one alternated and one eclipsed. In the eclipsed a hydrogen of the methyl is faced with the hydrogen of the hydroxyl group. The alternating has said hydrogens separated by an angle of 60º.
c) For methanol, two conformations can be drawn, one alternated and one eclipsed. In the eclipsed a hydrogen of the methyl is faced with the hydrogen of the hydroxyl group. The alternating has said hydrogens separated by an angle of 60º.