In nature, compounds with cycles of five and six links are very abundant. However, the three- and four-membered cycles appear very rarely in natural products.
Stability in cycloalkanes
These experimental facts suggest the greater stability of the cycles of five or six members with respect to those of three or four.
In the year 1885, the German chemist Adolf von Baeyer proposed that the instability of small cycles was due to the tension of the bond angles. The sp3 carbons have natural bond angles of 109.5º, in cyclopropane these angles are 60º, which is a deviation of 49.5º. This deflection translates into stress, which causes instability in the molecule.
Cyclobutane is more stable since its bond angles are 90º and the deflection is only 19.5º. Baeyer applied this reasoning to the other cycloalkanes and predicted that cyclopentane should be more stable than cyclohexane.
Angle strain
Observe the bond angles of the different cycloalkanes:
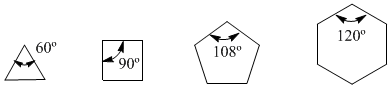
Since the natural angle of an sp3 carbon is 109.5º, von Baeyer reasoned that the most stable cycloalkane was cyclopentane. However, we know that Baeyer was wrong since the lowest energy (most stable) cycloalkane is cyclohexane. Bayer's error is in assuming that cycloalkanes are planar and that the only type of strain they present is due to bond angles.
Ring strain types
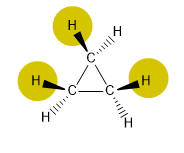
There are three types of strain that destabilize cyclic compounds:
1.- Bond angle tension, due to angles that differ from 109.5º.
2.- Eclipsing tension, due to nearby atoms or groups of atoms, which suffer repulsions (steric tension).
Cyclopropane presents a very important tension, due to the low bond angles and the interactions between hydrogens (eclipsations).