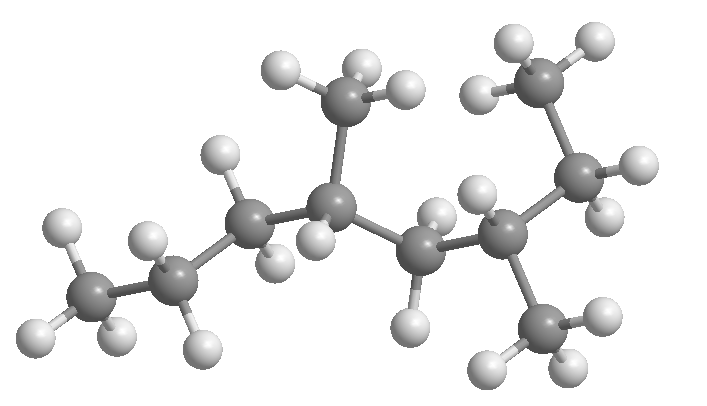
What are alkanes?
Alkanes are compounds formed exclusively by carbon and hydrogen (hydrocarbons), which only contain simple carbon-carbon bonds.
Types of alkanes
Alkanes are classified as linear, branched, cyclic, and polycyclic.
Alkanes nomenclature
Alkanes are named by ending in -ane the prefix that indicates the number of carbons in the molecule (methane, ethane, propane...)
Physical properties of alkanes
The melting and boiling points of alkanes are low and increase as the number of carbons increases due to interactions between molecules by London forces. Linear alkanes have higher boiling points than their branched isomers.
Conformational isomers
Alkanes are not rigid due to the spin around the C-C bond. The multiple shapes created by these rotations are called conformations.
Newman projection
The energy of the different conformations can be seen in the Newman projections. Thus, in the case of ethane, the eclipsed conformation is the one with the highest energy, due to the repulsions between hydrogens.
Potential energy diagrams
The different conformations of alkanes can be represented in a potential energy diagram, where we can see which conformation is more stable (minimum energy) and the energy needed to go from one conformation to another.
Combustion of alkanes
Given their low reactivity, alkanes are also called paraffins. The most important reactions of this group of compounds are radical halogenations and combustion. Combustion is the combination of hydrocarbon with oxygen to form carbon dioxide and water.