Name the following alcohols using IUPAC rules 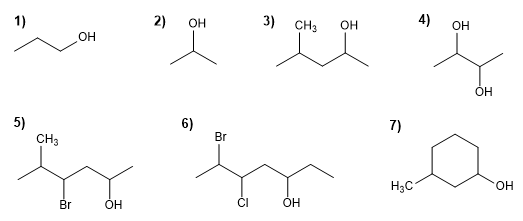
SOLUTION:
Molecule 1.
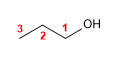
1. Main chain: the longest that contains the -OH (propane)
2. Numbering: Gives -OH the lowest locant.
3. Substituents: no
4. Name: Propan- 1 - ol
Molecule 2.
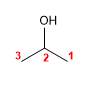
1. Main chain: the longest that contains the -OH (propane)
2. Numbering: indifferent.
3. Substituents: no
4. Name: Propan- 2 - ol
Molecule 3. 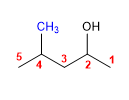
1. Main chain: the longest that contains the -OH (pentane)
2. Numbering: gives -OH the lowest locant ( -OH preferred over chains)
3. Substituents: 4-methyl
4. Name: 4 - Methyl pentan- 2 - ol
Molecule 4. 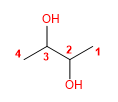
1. Main chain: longer length (butane)
2. Numbering: start at one end.
3. Substituents : no
4. Name: Butane- 2,3 -diol
Molecule 5.
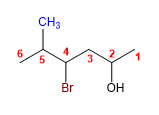
1. Main chain: longer length (hexane)
2. Numbering: Start at the far right, to give the -OH the lowest locant.
3. Substituents: bromo in position 4 and methyl in 5 .
4. Name: 4 - Bromo - 5 - methyl hexan- 2 - ol
Molecule 6. 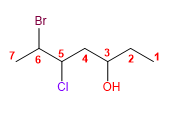
1. Main chain: longer length (heptane)
2. Numbering: starts at the end that gives the lowest locant to the -OH .
3. Substituents: 6 -bromo and 5 -chloro.
4. Name: 6 - Bromo - 5 - chloro hept- 3 - ol
Molecule 7. 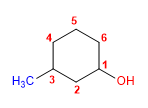
1. Main chain: six-membered cycle (cyclohexane)
2. Numbering: start at the -OH carbon.
3. Substituents: 3 -methyl.
4. Name: 3 - Methyl cyclohexanol ![]() 1. When there is more than one -OH group in a molecule, the quantity prefixes di, tri, tetra, penta, hexa,... can be used. The numbering must give the smallest locants to the -OH.
1. When there is more than one -OH group in a molecule, the quantity prefixes di, tri, tetra, penta, hexa,... can be used. The numbering must give the smallest locants to the -OH.
2. The name of the alcohol is built starting with the substituents, preceded by their respective locants, ending with the name of the main chain. The ending -o of the corresponding alkane is replaced by -ol.
3. In the case of cyclic alcohols it is not necessary to indicate the position of the hydroxyl group, since it always takes locant 1.