Name the following molecules, in which alcohol acts as a substituent. 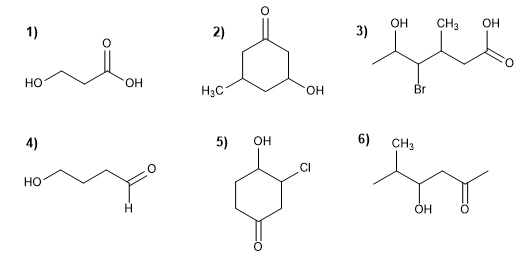
SOLUTION:
Molecule 1.
1. Main chain: longest that contains the functional group (propane)
2. Functional group: carboxylic acid
3. Numbering: lowest locant to the acid group
4. Substituents: 3- hydroxy group.
5. Name: 3 - hydroxy propanoic acid
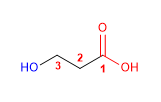
Molecule 2. 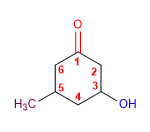
1. Main chain: six-membered cycle (cyclohexane)
2. Functional group: ketone
3. Numbering: lowest locant to the carbonyl group
4. Substituents: 3- hydroxy and 4 - methyl group.
5. Name: 2 - Hydroxy - 5 -methylcyclohexanone
Molecule 3. 
1. Main chain: longest that contains the functional group (hexane)
2. Functional group: carboxylic acid
3. Numbering: Assign the lowest locant to the acid group.
4. Substituents: 4 - bromo , 5 - hydroxy , and 3- methyl
5. Name: 4 - Bromo - 6 - hydroxy - 3 - methyl hexane oic acid
![]() Carboxylic acids and ketones take precedence over alcohols.
Carboxylic acids and ketones take precedence over alcohols.
The alcohol becomes one more substituent of the molecule, ordered alphabetically with the rest of the substituents.
Molecule 4.
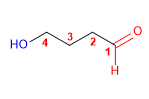
1. Main chain: longest that contains the functional group (butane)
2. Functional group: aldehyde
3. Numbering: lowest locant to the carbonyl group
4. Substituents: 4- hydroxy group.
5. Name: 4 - Hydroxy butane al
Molecule 5.
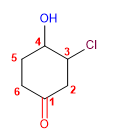
1. Main chain: six-member cycle
2. Functional group: ketone
3. Numbering: lowest carbonyl locant
4. Substituents: 3 - chloro and 4 - hydroxy .
5. Name : 3 - Chloro - 4 - hydroxy cyclohexanone
Molecule 6.
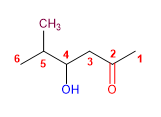
1. Main chain: longest that contains the functional group (propane)
2. Functional group: ketone
3. Numbering: lowest locant to the carbonyl group
4. Substituents: 4- hydroxy and 5 - methyl group.
5. Name: 3 - Hydroxy - 4 - methyl hexan- 2 - one