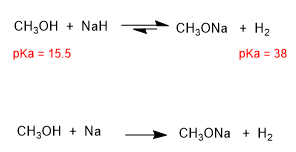
Alkoxides are the bases of alcohols, they are obtained by reacting alcohol with a strong base.
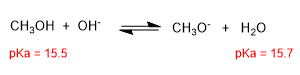
The pKa of the conjugate acids are similar and the equilibrium is not shifted. The hydroxide ion is too weak a base to form the alkoxide in any significant amount.
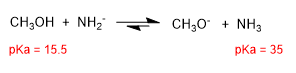
The amide is a very strong base and shifts the equilibrium to the right, transforming methanol into methoxide.
Other strong bases that can be used to form alkoxides are: sodium hydride, LDA, sodium metal.