The reaction between two different carbonyls is called crossed or mixed aldol. This reaction only has synthetic utility in two cases:
1. Only one of the carbonyls can form enolates.
2. One of the carbonyls is much more reactive than the other.
In all other situations, the mixed aldol generates mixtures of four products. Let's see as an example the condensation of ethanal and propanal. 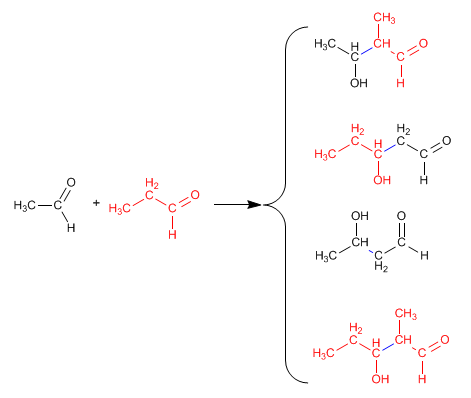
The mixed aldol condensation of ethanal with benzaldehyde generates a product, when working in excess of benzaldehyde, because benzaldehyde lacks hydrogens on the alpha carbon and cannot form enolates. 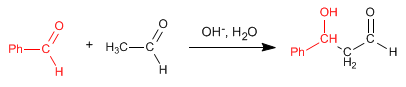
The mechanism of this reaction takes place in the following stages:
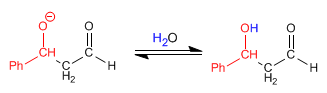
Stage 1. Ethanal enolization 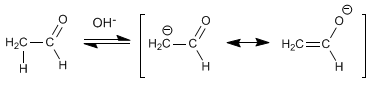
Enolate formation can only take place with ethanal, since benzaldehyde lacks acidic hydrogens on the alpha carbon.
Stage 2. Nucleophilic attack of the enolate on benzaldehyde. 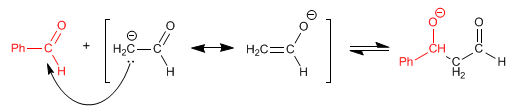
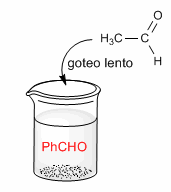 At this stage, the attack of the ethanal enolate on itself can occur.
At this stage, the attack of the ethanal enolate on itself can occur.
same. To avoid this, work in excess of benzaldehyde. A widely used experimental procedure to avoid the condensation of ethanal with itself is to slowly drop the ethanal on a basic solution of benzaldehyde.
Stage 3. Protonation