Aldehydes and ketones react in an aqueous acid medium to form hydrates. The mechanism consists of three stages. The first and fastest is the protonation of carbonyl oxygen. This protonation produces an increase in polarity on the carbon and favors the attack of the nucleophile. In the second stage, the water attacks the carbonyl carbon, it is the slow stage of the mechanism. In the third stage, oxygen deprotonation occurs, forming the final hydrate.
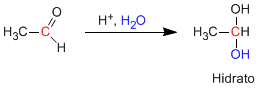
Reaction mechanism
Stage 1. Protonation of carbonyl oxygen. 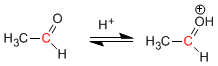
Stage 2. Nucleophilic attack of water on the protonated carbonyl. 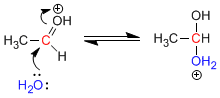
Stage 3. Deprotonation of the hydrate 
Forum: Aldehydes and ketones