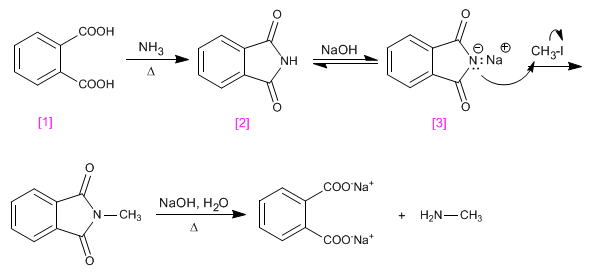
The Gabriel synthesis allows primary amines to be obtained from haloalkanes, without the formation of mixtures of secondary and tertiary amines.
Gabriel starts from benzene-1,2-dicarboxylic acid, which by reaction with ammonia produces Phthalimide. The basic treatment of Phthalimide generates its salt, which is alkylated by reaction with the haloalkane. A final hydrolysis of the imide leaves the primary amine and the benzene-1,2-dicarboxylic acid salt free.