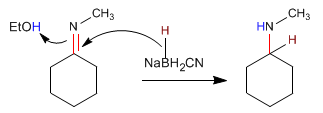
Reductive amination consists of forming an imine, from aldehydes or ketones and amines, which is reduced to an amine in a subsequent stage. This reduction can be carried out with $H_2$ catalyzed by Nickel or with $NaBH_3CN$.
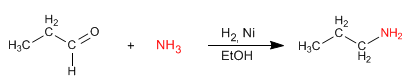
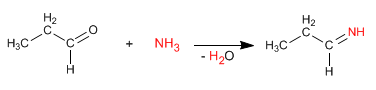
The reaction proceeds with formation of imine from propanal and ammonia.
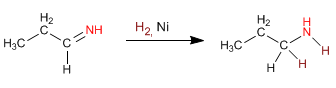
The imine double bond is reduced with H 2 , Ni, to form the final amine.
Let's see a second example
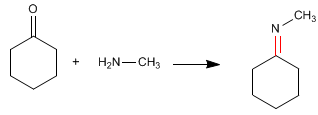
In the first stage, the imine is formed from cyclohexanone and methylamine.
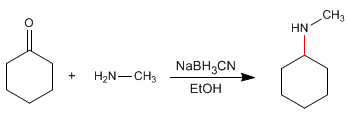
In the second stage, sodium cyanoborohydride reduces the imine to an amine.