Hard nucleophiles (organometallic, amide, lithium aluminum hydride) attack position 2 of the pyridine ring, giving rise to nucleophilic addition reactions. In the event that a final oxidation stage occurs, with loss of hydride, we can speak of nucleophilic substitution.
a) Addition of organometallics
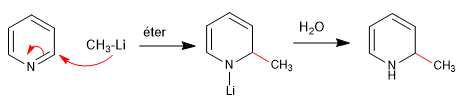
Rearomatization of the ring is possible by adding an oxidant that removes "H 2 "
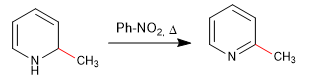
b) Addition of amide. Chichibabin's reaction
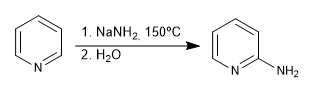
Mechanism:
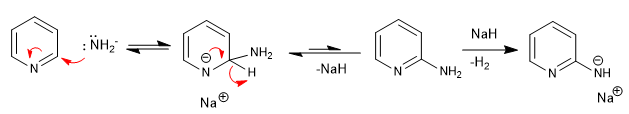
The sodium amide formed is protonated in the final stage of aqueous workup.
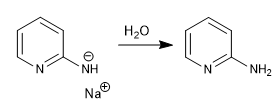
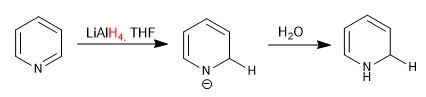
c) Addition of hydrides
Lithium aluminum hydride adds hydrides to the 2-position of pyridine.
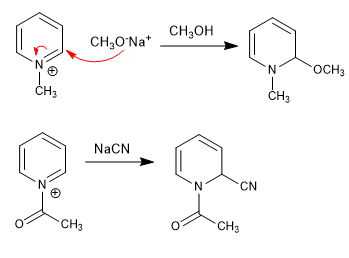
The weaker nucleophiles (cyanide, hydroxide, methoxide) fail to attack the 2-position of the pyridine, but do react on pyridinium salts, thanks to the greater polarity of the 2-position.