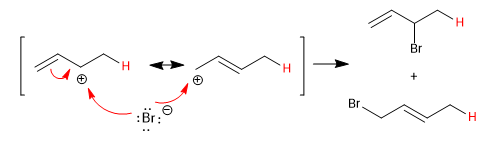
The conjugated dienes add the acids of the halogens forming kinetic and thermodynamic products, whose ratio can be controlled with the reaction conditions (temperature and time).
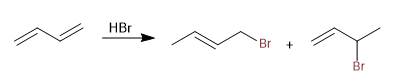
Mechanism:
Stage 1. Electrophilic addition with formation of allylic carbocation
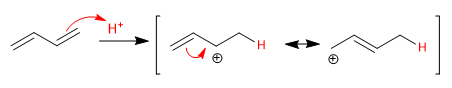
Stage 2. Nucleophilic attack of the bromide.