SYNTHESIS OF ALCOHOLS
(Synthesis Tree Method)
Propose a synthesis plan for the indicated target molecules from the indicated single molecules (MOb 30 -41). To do this, use the reagents and reaction conditions that you think are necessary:
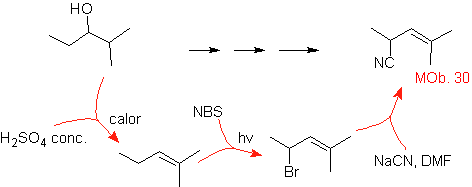
MOb 30 solution.
Strategy: It is observed that the starting molecule has been dehydrated and in the initially unsubstituted allylic position, a hydrogen has been displaced by the cyano or nitrile group. This last reaction can occur only if the precursor molecule is an allylic halide, which is why it is proposed as a precursor of
The Br is introduced in the desired position with the NBS and the alkene is the product of the dehydration of the starting molecule.
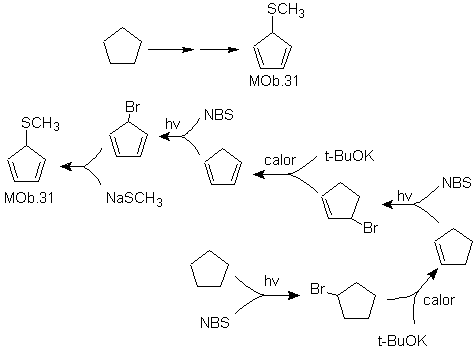
Solution MOb 31.
Strategy : It is a uncle ether, the necessary precursor molecule will be a 1,3-cyclopentadiene halide.
This halide is prepared by the action of NBS on the dienic cycloalkene, which in turn is prepared by the dehydrobromination of the precursor molecule, which is reached by the action of NBS on the cycloalkene formed. previously by dehydrohalogenation of the starting molecule brominated by radicals
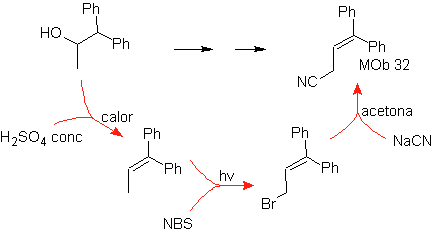
Mob 32 solution.
Strategy : It is similar to the one used in obtaining
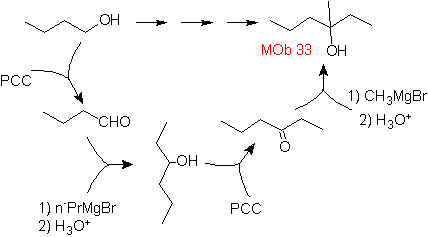
Solution MOb 33.
Strategy :
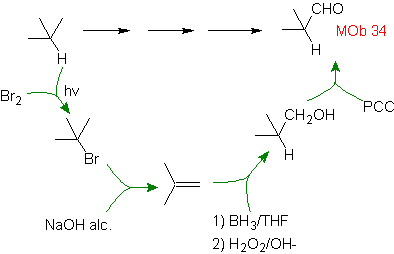
Solution MOb 34.
Strategy :
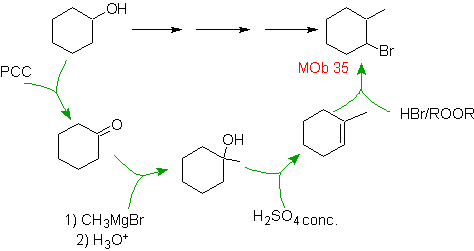
MOb 35 solution.
Strategy : An alkene is proposed as a precursor molecule, which is brominated under antimarkovnikov conditions. The alkene was prepared by dehydration of the alcohol formed, by the action of a Grignard on a ketone, which is prepared by oxidation of cyclohexanol.
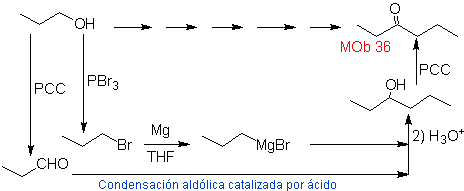
Solution MOb 36.
Strategy:
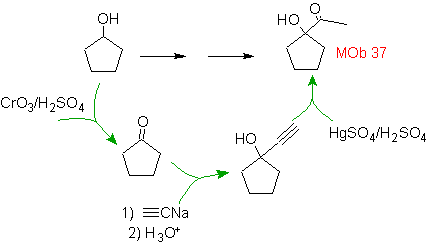
Solution MOb 37.
Strategy: The methyl ketone of
The alcohol was formed by the action of sodium acetylide on a ketone derived from the starting alcohol by oxidation.
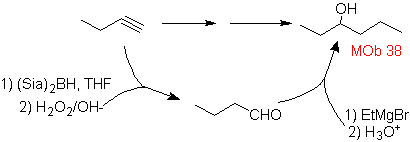
Mob solution 38.
Strategy: The starting material is oxidized to aldehyde with disiamilborane, which attacked by a suitable Grignard to form
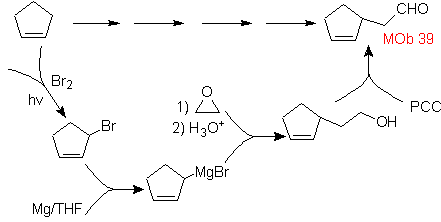
Mob solution 39.
Strategy : As
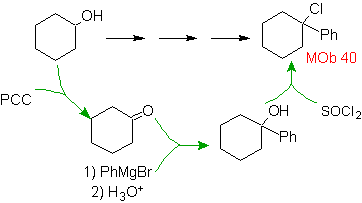
MOb 40 solution.
Strategy : The halides are obtained from alkenes or alcohols, the latter is the best option, because the action of a Grignard group that contains the phenyl group allows the alcohol required to be formed as a precursor molecule.
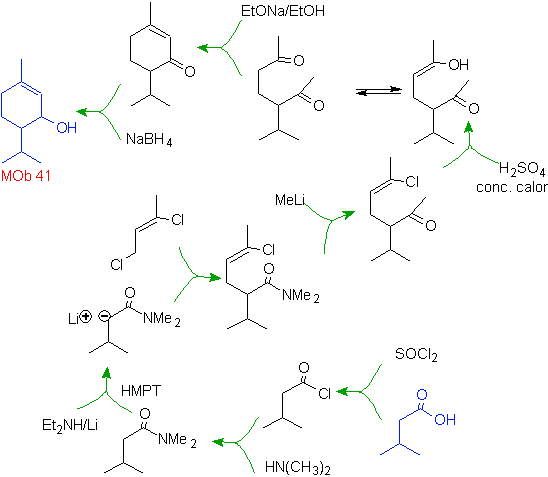
The following two syntheses require a little more effort from the chemist, because the defined starting materials require the use of some reactions that are not the most common, despite the fact that the structures of the target molecules are relatively simple.
So how is the formation of molecules 41 and
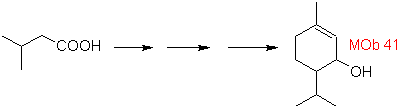
On the other hand, methyl lithium in stoichiometric amounts leads to methyl ketone only when the substrate is an amide or a free acid (J. Organometal Chem., 9, 165 (1967). Alternatively, the use of methyl magnesium iodide is also suggested in place of methyllithium.
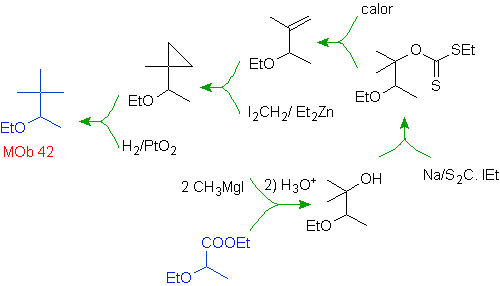
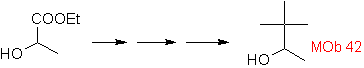
Mob 42 solution.
The opening of the cyclopropane molecule, a precursor to