DISCONNECTIONS OF 1,3-DIOXYGEN COMPOUNDS
Oxygenated organic compounds are the most abundant in nature and particularly dioxygenates, which is why many chemical researchers have modeled the retrosynthetic disconnection of these molecules, an aspect that will be studied in the following paragraphs.
To begin with, the disconnection models of dioxygenated molecules have been divided into two large groups, based on the nature of the synthons that are generated with the application of a basic synthetic operation called " DISCONNECTION" to the molecule to be synthesized and that generally it is referred to as a target molecule (MOb) .
These large groups are:
![]() “ Logical ” disconnection models, and
“ Logical ” disconnection models, and
![]() “ Anomalous ” or “ illogical ” disconnection models
“ Anomalous ” or “ illogical ” disconnection models
The so-called "logical" disconnection models are those that, by applying a "disconnection" of one or several chemical bonds in
Compounds that can be classified as 1,3-dioxygen and 1,5-dioxygen, when subjected to retrosynthesis, generally form synthons considered "logical." On the other hand, organic molecules related to 1,2-dioxygenated, 1.4-dioxygenated and 1,6-dioxygenated, generate synthons
considered “illogical”
Cut-off model 1,3-dioxygen
![]() β-dicarbonyl compounds
β-dicarbonyl compounds
The 1,3-dicarbonyl compounds are obtained with good yields through Claisen-type condensation reactions, which involve the reaction between esters and compounds with active hydrogens, such as: esters, ketones, aldehydes, nitriles, nitroderivatives, and some hydrocarbons in presence of alkaline reagents.
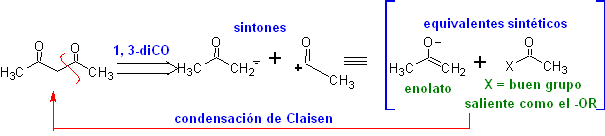
The β-diketones and β-ketoaldehydes are obtained by the crossed Claisen condensation, using a suitable ketone and ester. In the crossed Claisen condensation of ketones and esters, good yields are obtained because ketones are notably more acidic than esters, therefore, in the basic medium, the ketone is deprotonated to a greater degree than the ester.
Examples : Propose a synthesis design from simple materials, for the following molecules:
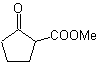
MOb 16
| mob 17
| MOb 18
|
Solution:
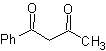
MOb 16 . Apparently the two disconnection alternatives (a) and (b), shown in
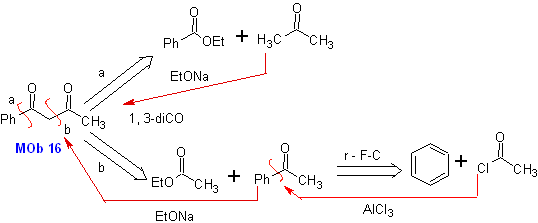
However, alternative (b) turns out to be the most suitable, since, in the basic reaction medium, the carbanion formed PhCOCH 2 - would be better stabilized, due to resonance and inductive effects.
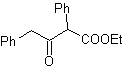
MOb 17. Disconnection (a), in
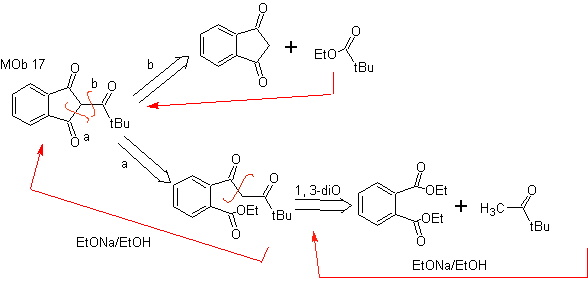
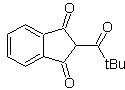
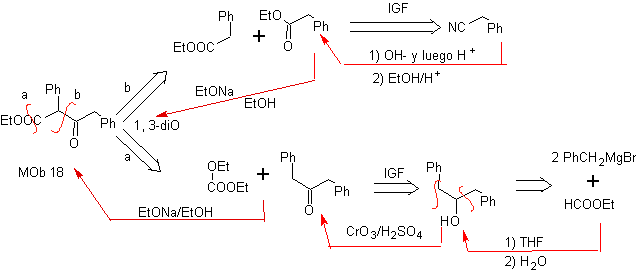
MOb 18. The best disconnection alternative in this MOb is (b), because it leads to symmetrical synthetic equivalents. the alternative (a) is not without its importance, in the use of ethyl formate.
![]() β-Dicarbonyl compounds through intramolecular condensations
β-Dicarbonyl compounds through intramolecular condensations
Propose a feasible synthesis plan for the following molecules: | mob 19
| MOb 20
|
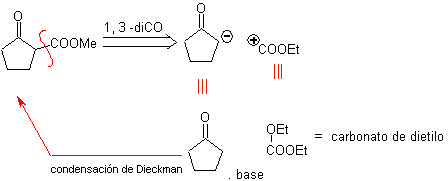
MOb 19. The disconnection 1,3–diCO, leads to a diester in relation 1,6. They are easy to reconnect to an alkene hexacycle, a model that will be studied a little later. For this reason, an alternative synthesis design is provided, based on known reactions.
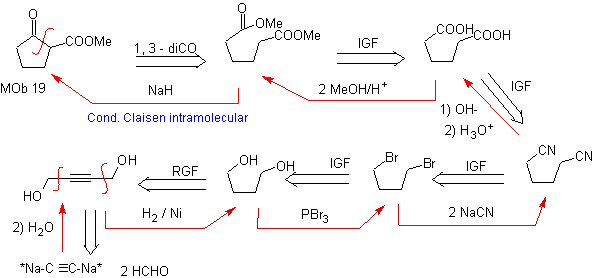
Design that can only be questioned by the large number of steps, which generally decreases the yield of the synthesis.
It is possible to think of another disconnect 1, 3 – diCO for
MOb 20 . Sometimes it is necessary to exercise control on the structure of
So,
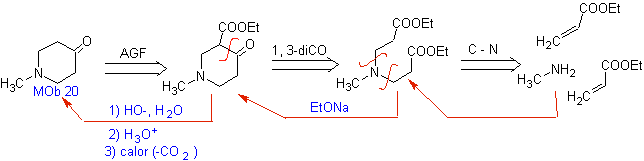
![]() beta -Hydroxy compounds
beta -Hydroxy compounds
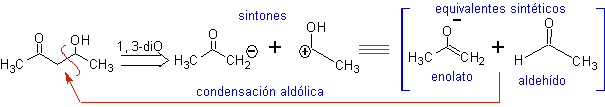
MOb21 . The best disconnection in beta-hydroxy compounds is by the bond formed between C to
and C b , relative to the carbonyl group. These types of compounds are typical products of aldol condensation reactions and the like, which is why a good synthesis design for
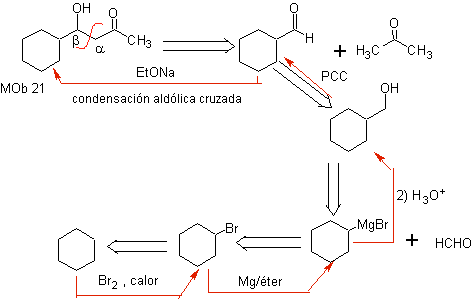
The base to be used to form the b -hydroxycarbonyls must be weak, to avoid dehydration of the alcohol function and thus produce an a , b unsaturated carbonyl compound, which will be the object of our study in the next paragraph.
The reaction between formal-cyclohexanone and acetone in a basic medium is a condensation reaction of the aldol type. The aldehyde group is the most reactive and there is no risk of self-condensation of the aldehyde by steric effects.
![]() a , b unsaturated carbonyl compounds ( a , b insat.CO )
a , b unsaturated carbonyl compounds ( a , b insat.CO )
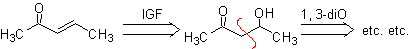
The α, β-unsaturated carbonyl compounds are easy to prepare by dehydrating β-hydroxycarbonyl compounds, which is why their disconnection involves functionalizing the unsaturated molecule to an alcohol. The olefinic bond could also be prepared using the Wittig reaction.
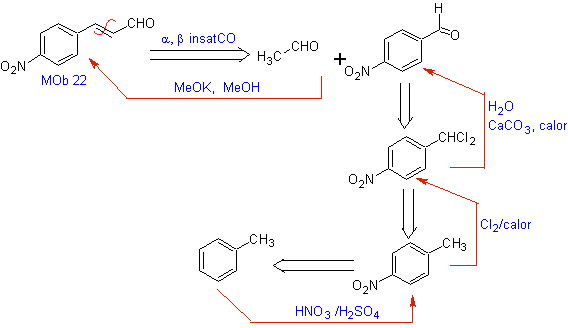
MOb 22. Since an a,b unsaturated carbonyl compound , MOb 22 , can be functionalized to the corresponding alcohol, to obtain a 1,3-dioxygenated model, it is possible to disconnect the molecule directly by the double bond, formulating a C=O group on carbon b and a -CH 3 group on carbon a .
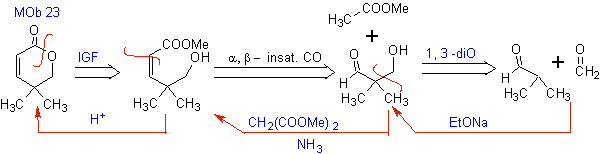
MOb 23. The disconnection of this molecule begins with the cyclic ester (lactone), as it is the most critical point, which then allows it to be disconnected as an a , b unsaturated carbonyl compound.
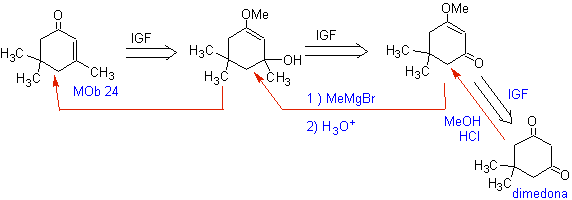
MOb 24. The conventional disconnection by the double bond of