Thus, 2,6-heptanedione condenses with methoxide in methanol to form 3-methylcyclohex-2-enone.
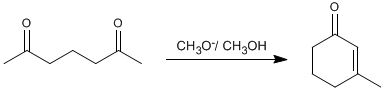
The reaction mechanism proceeds through the following stages:
Stage 1. Formation of the enolate.
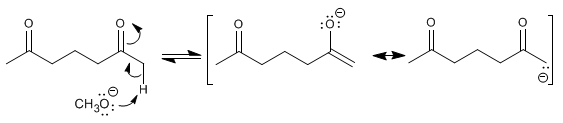
Step 2. Intramolecular nucleophilic addition
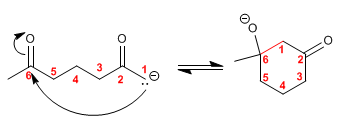
Step 3. Protonation of the aldol base
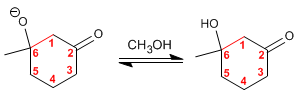
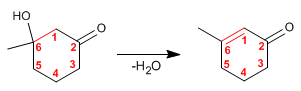
Step 4. Dehydration of the aldol