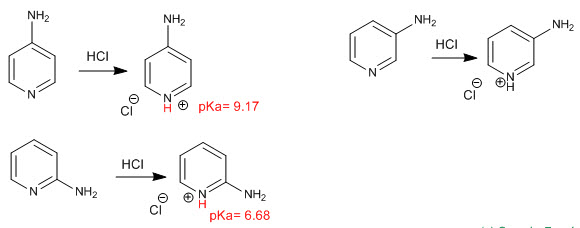
Pyridine behaves as a base through the lone pair of the nitrogen atom. In the presence of acids, it is protonated, generating pyridinium salts.
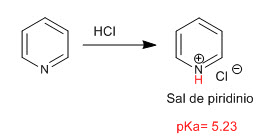
The basicity of nitrogen is modified by the presence of substituents. Thus, the groups that give up charge by resonant effect (mesomer) tend to increase the basicity of nitrogen, while those that steal charge by resonant effect decrease the basicity.
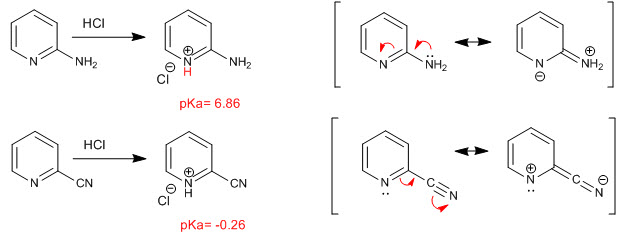
As can be observed in the pKas, the amino increases the basicity of the nitrogen by transferring the free pair towards the heteroatom. However, the cyano group steals charge, both by inductive effect and by resonance of the annular nitrogen, considerably decreasing its basicity.
The position of the substituent within the ring also determines the degree of basicity of the nitrogen, with great differences between the basicity of the three amino pyridines.