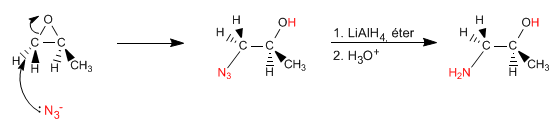
Epoxides (oxacyclopropanes) open by nucleophile attack, due to the significant ring strain. If the nucleophile used is ammonia, a β-aminoalcohol is obtained. This type of product can also be obtained by opening the epoxide with sodium azide, and reducing the azide in a second stage.
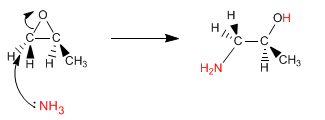
The opening of the epoxide takes place on the least substituted carbon, as it is a basic medium.
The epoxide can also be opened with sodium azide, reducing the azide formed with lithium aluminum hydride.