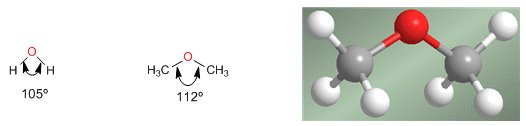
Ethers are molecules similar in structure to water and alcohols. The angle between the C-O-C bonds is greater than in water due to steric repulsions between bulky groups.
In the case of epoxides, the most relevant characteristic is the ring tension due to bond angles very distant from 109º, the C-O-C bond presents an angle of 61º.
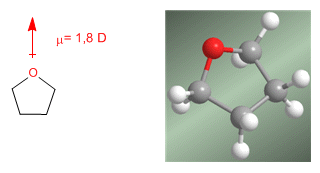
Ethers are very polar molecules. Thus, Diethyl ether presents a dipole moment of 1.2 D. This dipole moment is even more important in cyclic ethers (oxacyclopropane, tetrahydrofuran) that present dipole moments above 1.8 D, similar to water.