The instrument that allows detecting the interaction between electromagnetic radiation and matter is called a spectrophotometer and its basic structure can be seen in the following diagram.
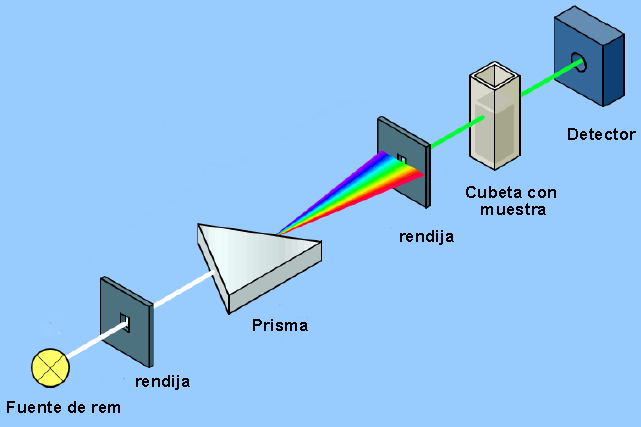
The spectrophotometer consists of a light source (bulb) that produces white light. The first slit selects a beam that contains all the emitted frequencies. This ray passes through a glass prism that breaks down white light into its different frequencies (from red to violet). A second slit selects one of the frequencies (monochromatic light) that will fall on the cuvette containing the sample. The set of the prism and the second slit is called a monochromator.
The monochromatic ray passing through the sample hits the detector, which transfers the data to a computer system where the spectrum is generated. If the frequency selected in the second slit is not absorbed by the sample, a baseline point of the spectrum is produced. When the frequency of the radiation is adequate to produce a transition (vibrational, electronic...) an absorption peak is observed in the spectrum.
Spectrum analysis makes it possible to determine the structure of the molecule that produces it.