The determination of the structure of an organic molecule begins with the analysis of the elements it contains and their proportion, which is usually done by combustion. Molecular mass determination, previously performed by cryoscopic descent, now uses the high-resolution mass spectrometry technique.
Knowledge of the percent composition and molecular mass allow us to establish the molecular formula of an organic compound. However, two fundamental steps are still missing, which are determining connectivity and spatial arrangement.
a) Connectivity refers to indicating the way in which the different atoms that form an organic molecule are joined. This level of description is done in the plane, without taking into account the spatial arrangement of the molecule.
Let's consider the molecular formula C 3 H 6 O 3 , let's see different forms of connectivity, which give rise to the so-called structural isomers.
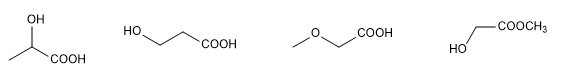
All these molecules have the formula C 3 H 6 O 3 but the atoms are joined in a different way in each of them.
b) Now let's look at the first molecule. We observe that carbon 2 has four different substituents (CH 3 -, H-, -OH, -COOH), which makes it chiral or asymmetric. These four substituents can be spatially arranged in two ways that give rise to two molecules, mirror images of each other.
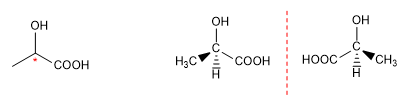
These molecules that have the same connectivity but differ in the spatial arrangement of groups, both being mirror images, are called enantiomers.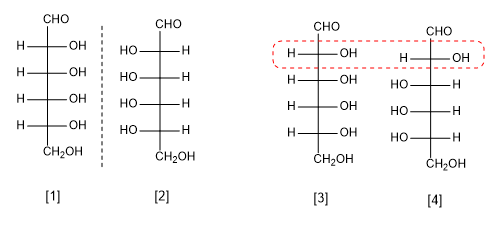
Spatial isomers (stereoisomers) that are not mirror images are called diastereoisomers.

y : pair of enantiomers
y Pair of diastereoisomers