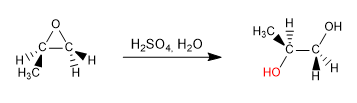
Epoxides (oxacyclopropanes) are three-membered cyclic ethers. Its main characteristic is the tension of the ring, which favors its opening in both basic and acidic media.
Opening in basic media: Epoxides open by nucleophile attack on the least substituted carbon (opening is governed by steric hindrances)
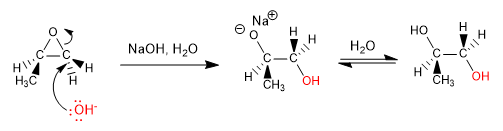
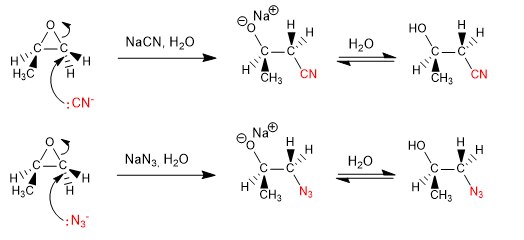
Other examples of opening in basic medium:
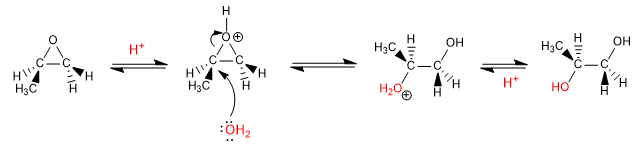
Opening of epoxides in acid media: In acid media, oxygen protonation occurs, favoring its capacity as a leaving group and increasing the polarity of the carbons to which it binds. Under these conditions, the nucleophile attack occurs on the most substituted carbon.
Mechanism: