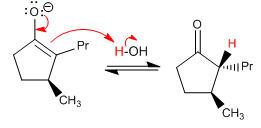
Treating cis -2-propyl-3-methyl-cyclopentanone with a weak base transforms it into its trans stereoisomer. Reason this phenomenon and draw the corresponding structures.
SOLUTION:
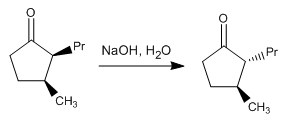
Stage 1 . Enolate formation
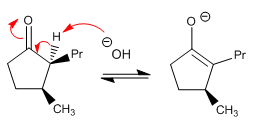
Stage 2. Protonation of the enolate on the top face to reduce repulsions between groups