Explain why when a solution of (S)-3-phenyl-2-butanone in aqueous ethanol is treated with acids or bases it gradually loses its optical activity. (b) Why does the racemization of the compound from the previous section in an acid medium occur at the same speed as the halogenation in an acid medium? (c) Why does the iodination of said compound in an acid medium occur at the same rate as acid-catalyzed bromination?
SOLUTION:
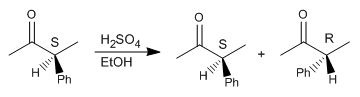
Step 1. Protonation of the carbonyl
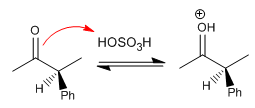
Stage 2. Formation of the enol (Slow Stage)
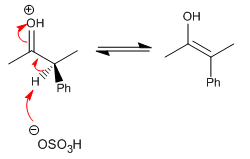
Stage 3. Protonation of the enol with formation of the racemate
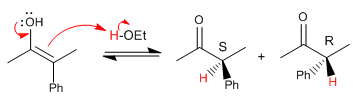
Now let's see the mechanism of halogenation with bromine or iodine of the alpha position of this carbonyl.
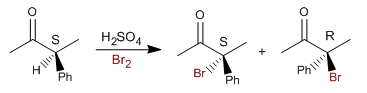
Step 1. Protonation of the carbonyl
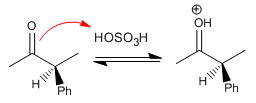
Stage 2. Formation of the enol (Slow Stage)
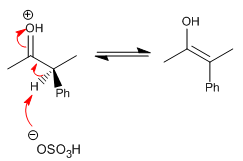
Stage 3. Nucleophilic attack of the enol on the bromine
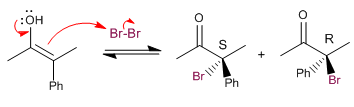
Racemization and halogenation present the same slow step (enol formation), taking place at the same speed.