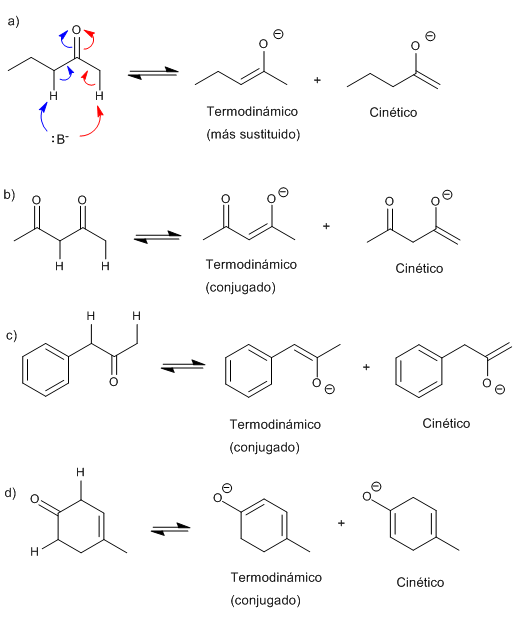
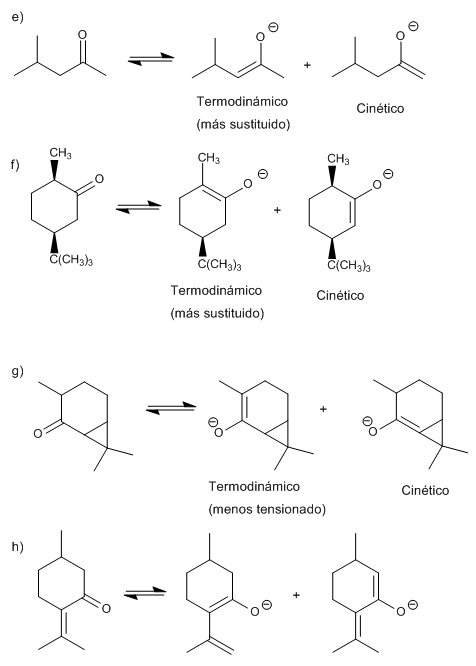
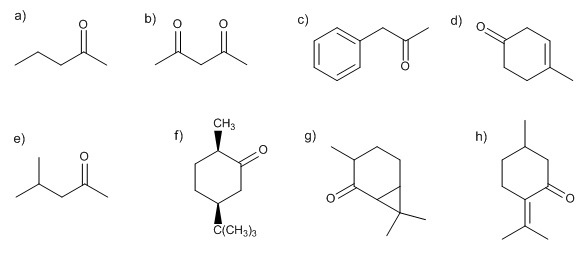
Write the structure of all the possible enolates of each ketone or aldehyde. Indicate which would be the most favored under kinetic or thermodynamic control. Explain.
SOLUTION:
The thermodynamic enolate is the most stable (more substituted or conjugated), it is formed using small and not very strong bases.
The kinetic enolate is formed by speed (unstable), using very bulky and strong bases.