In stereochemistry, asymmetric induction (also enantio-induction) in a chemical reaction describes the preferential formation of a enantiomer either diastereomer on the other, as a result of the influence of a characteristic chiral present in the substratum, reagent, catalyst or environment. Asymmetric induction is a key element in the asymmetric synthesis.
Asymmetric induction was introduced by Emil Fischer, based on his work on the carbs. There are several types of induction.
The internal asymmetric induction makes use of a chiral center linked to the reactive center by means of a covalent bond, and remains so during the reaction. In the asymmetric relay induction chiral information is introduced in a separate step, and removed again in a separate chemical reaction. The special synthons are called chiral auxiliaries. In the external asymmetric induction, chiral information is introduced into the transition state through a catalyst either chiral ligand. This method of asymmetric synthesis it is economically the most desirable.
Stereoselectivity in the nucleophilic addition reaction to carbonyls (Cram's Rules)
In nucleophilic addition reactions, the carbon atom of the carbonyl group can be transformed into an asymmetric carbon atom, depending on the type of nucleophile used and the alkyl radicals that are initially attached to the C=O.
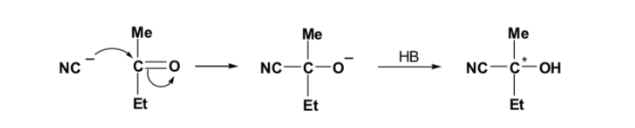
If the carbonyl compound and the nucleophile are not chiral, an equimolar mixture of two enantiomers (racemic) is obtained:
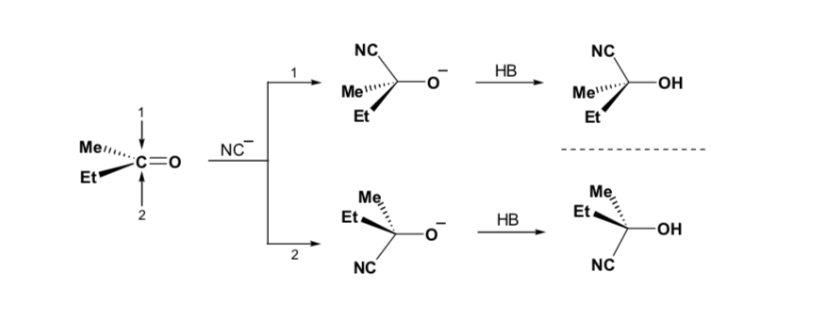
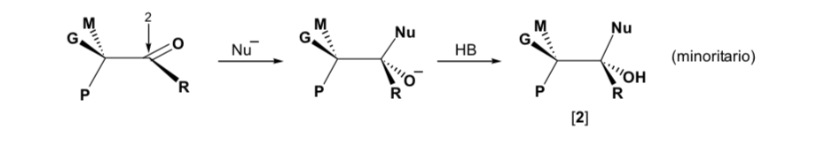
However, when the carbonyl compound is chiral, the probability that the nucleophile will react by each of the two faces of the carbonyl group is not the same. Stereo hindrance causes the reaction on one of the faces to be favoured, and the result is a mixture of diastereoisomers in different proportions ( asymmetric induction ):
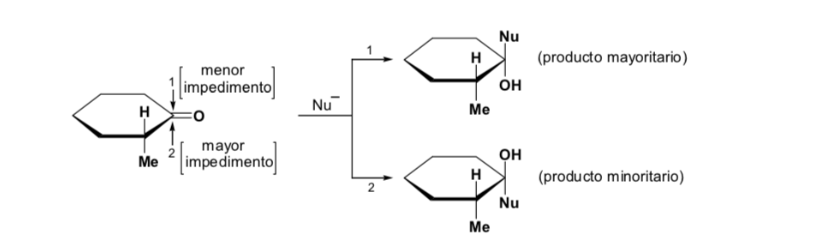
The major diastereomer is formed when the nucleophile reacts with the carbonyl group on the least hindered side, and the conformation of the substrate is one in which the carbonyl group is flanked by the less bulky groups attached to the asymmetric Cα.
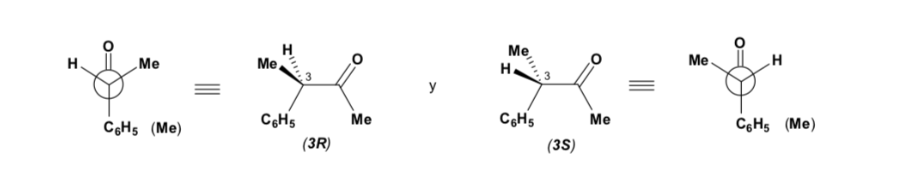
[carbonyl group flanked by the less bulky groups: H and Me ]
Cram's rule refers to the reaction of one of the stereoisomers that form the pair of enantiomers, not to the reaction of the racemic with the nucleophile.
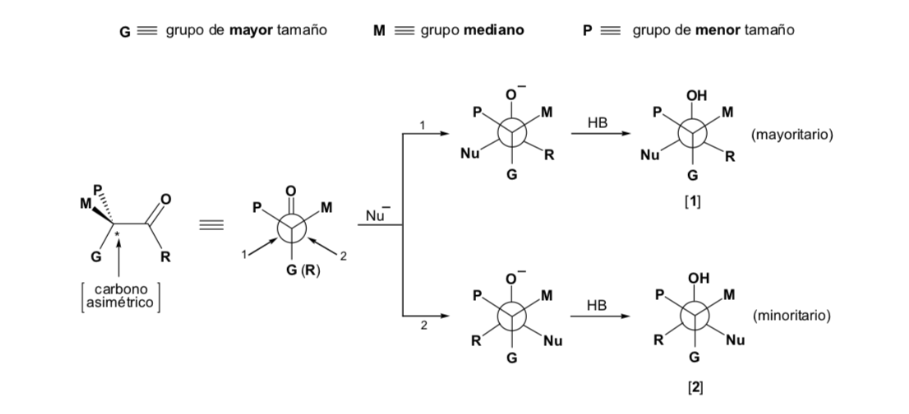
The nucleophile is approached from the side where P and G are located:
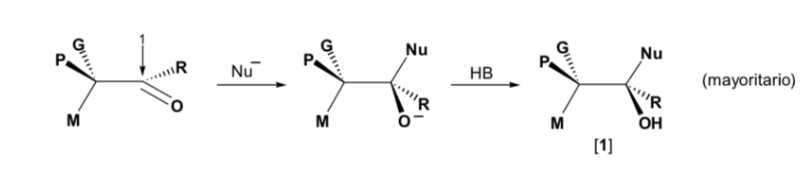
The nucleophile is approached from the side where M and G are located:

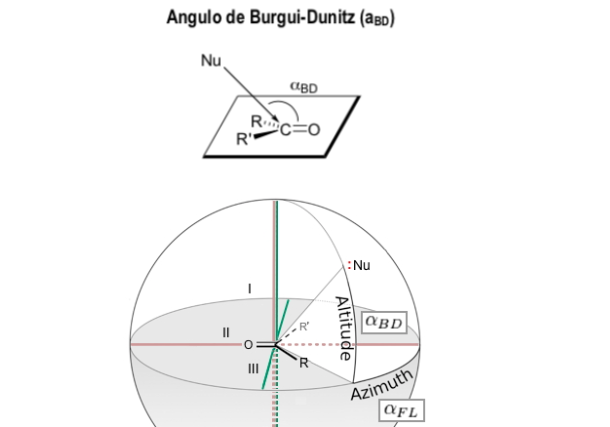
Burgi-Dunitz angle
The Bürgi-Dunitz angle (BD angle) is one of two angles that completely define the "attack" geometry (collision approximation) of a nucleophile on an unsaturated trigonal center in a molecule, originally the carbonyl center in an organic ketone, but now it is extended to aldehyde, ester, and amide carbonyls, and also to alkenes (olefins).
Zimmerman model - traxler
Zimmerman and Traxler proposed that the aldol reaction with metal enolates occurs via a chair-like pericyclic process. In practice, the stereochemistry can be highly dependent on the metal. Only a few metals, such as boron, reliably follow the indicated pathways.
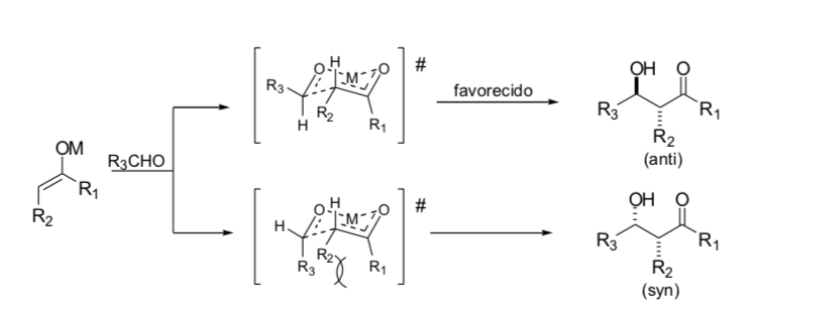
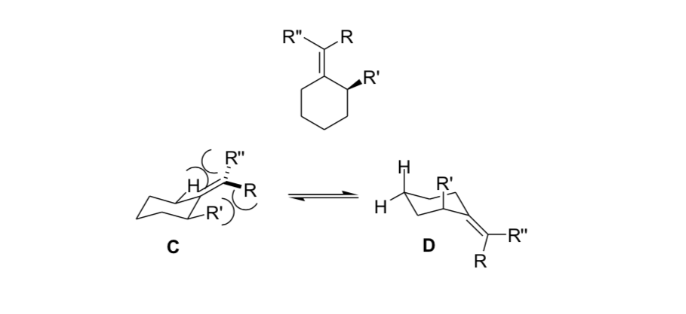
allylic stress
In stereochemistry, allylic tension or 1,3-allylic tension is a tension energy that results from an unfavorable molecular conformation for the allyl group, product of the interaction between a substituent at one end of an olefin with an allylic substituent at the other end.
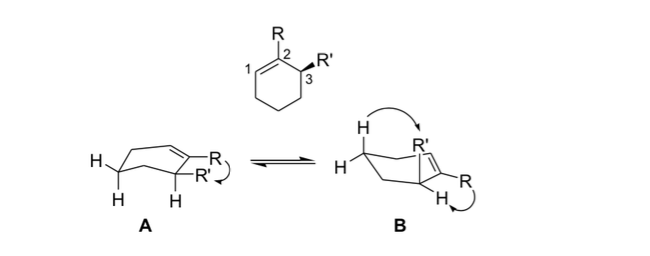
Organic chemists use this stiffness resulting from allylic strain to obtain asymmetric reactions.
Substitution at the alpha position of enolates: introduction of a new stereogenic center:
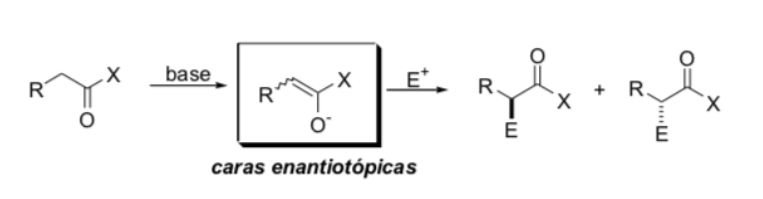
Factors controlling stereoselectivity
a) enolate geometry
b) sources of chirality present either in the enolate or in the electrophile (source of asymmetric information)
c) stereoelectronic effects
Sources to expand information:
1) Guo-Qiang Lin, Yue-Ming Li, Albert SC Chan. Principles and Applications of Asymmetric Synthesis. Ed. Wiley-Interscience. Great Britain, 2001.
2) Mark Rizzacasa and Michael Perkins. Stoichiometric Asymmetric Synthesis. Ed. Sheffield Academic Press. USA and Canada. 2000.
3) Jonathan MJ Williams. Catalysis in Asymmetric Synthesis. Ed. Sheffield Academic Press. USA and Canada. 1999.
4) RA Aitken and SN Kulényi. Asymmetric Synthesis. Ed. Blackie Academic and Professional. Great Britain, 1992.
5) Grossman RB The Art of Writing Reasonable Organic Reaction Mechanisms. Springer, New York. 2003
6) Norman ROC; Coxon JM Principles of Organic Synthesis. CRC Press, Boca Raton. 1993