Stereochemistry
Stereochemistry is the study of organic compounds in space. To understand the properties of organic compounds it is necessary to consider all three spatial dimensions. The bases of stereochemistry were laid by Jacobus van't Hoff and Le Bel, in the year 1874, as well as by Ernest L. Eliel in the 20th century. They independently proposed that the four substituents on a carbon point toward the vertices of a tetrahedron, with the carbon at the center of the tetrahedron.
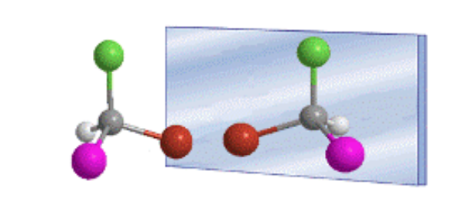
The tetrahedral arrangement of substituents on an sp 3 carbon gives rise to the existence of two possible compounds, which are non-superimposable mirror images, called enantiomers.
In general, molecules that differ by the spatial arrangement of their atoms are called stereoisomers.
Isomeria
Isomers are those compounds that have identical molecular formulas but that differ in the nature or arrangement of the bonds between their atoms or in the arrangement of their atoms in space.
To interpret differences in properties, chemists in the last century imagined that the atoms of a molecule had particular spatial arrangements that accounted for their different behaviors.
The classification by chemical function, established according to the behavior of the compounds, has been related to the presence in the molecule of a group of atoms called a functional group.
In addition to the importance of the functional group, there is a difference in behavior induced by slight differences in the arrangement of the different atoms that make up the rest of the molecule. These differences can respond to different classes of isomerism:
function isomerism
Constitutional isomers, which differ from each other in that their functional groups are different, belong to this type of isomerism.
The functional group in both isomers is different
C2H6O _
Ethanol (CH 3 -CH 2 -OH) and dimethyl ether (CH 3 -O-CH 3 )
Alcohol reacts with sodium while with ether no reaction is observed.
From a physical point of view, alcohol is a liquid with a boiling point of 78.5°C, while ether is a gas that liquefies at -23°C.
Position and/or skeleton isomerism.
The functional groups are identical but are placed in different positions on the molecular skeleton (positional isomers).
Ex: 2-hexanol and 3-hexanol:
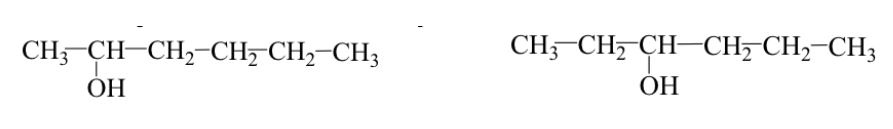
Sometimes the alkyl group has a different arrangement (backbone or branching isomers).
Ex: 3-methyl-2-pentanol and 2-hexanol
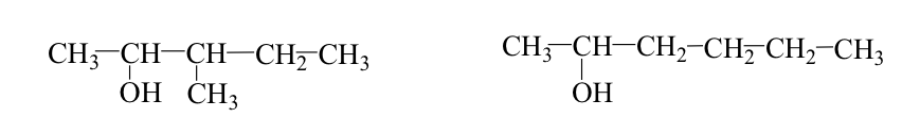
Both cases can occur simultaneously:
Ex: 3-methyl-2-pentanol and 3-hexanol
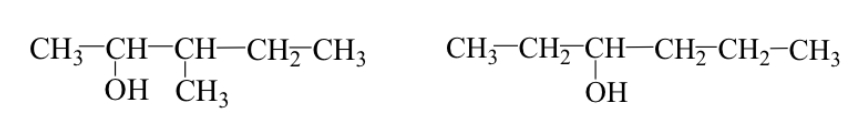
tautomerism
They are constitutional isomers of easy interconversion because they are in rapid equilibrium with each other. The phenomenon is called tautomerism and usually consists of an atom, usually hydrogen, located in a triad of atoms, and a double bond changing position simultaneously.
The most classic example is the keto-enol equilibrium ( -ene for the double bond and -ol for the alcohol).
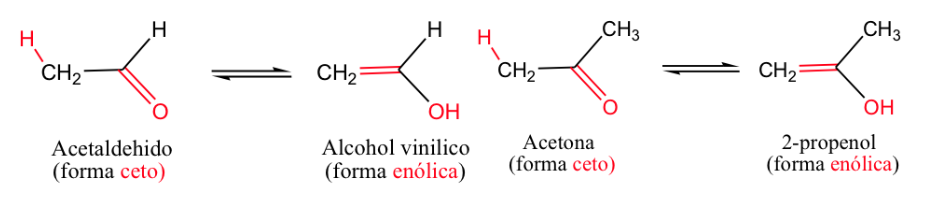
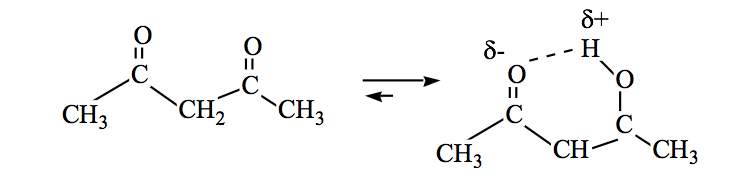
Generally, the ketone forms are the most stable, but when the enol form stabilizes (by hydrogen bonding or by resonance) the equilibrium shifts.
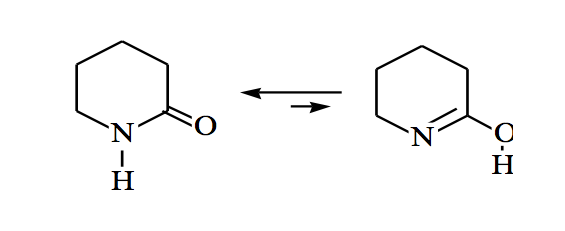
Amides can also be in keto-enol equilibrium:
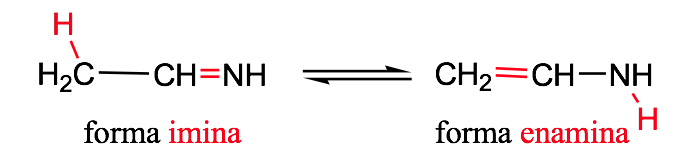
Imine-enamine tautomerism:
nitro-aci
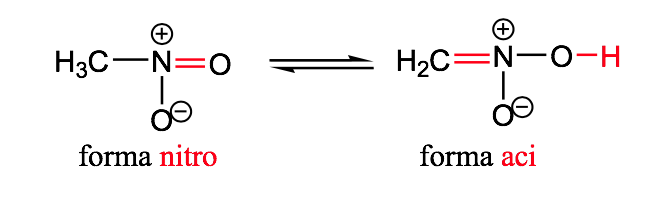
geometric isomerism
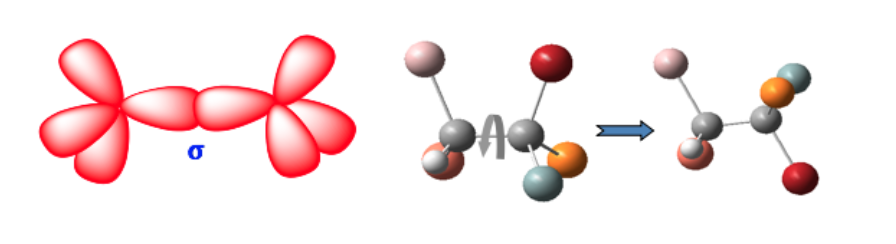
These two forms are not geometric isomers since free rotation around the single bond converts one form into another (conformers).
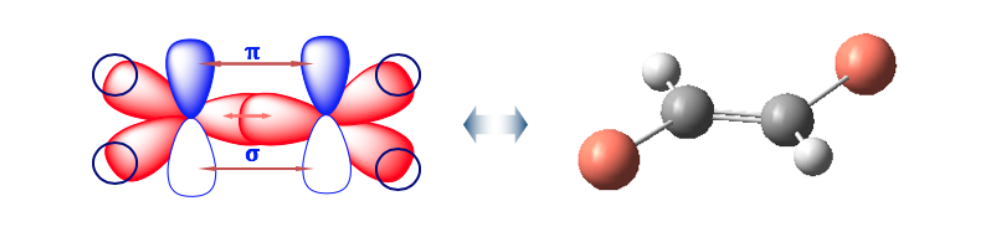
The double bond does not allow free rotation, which can generate two different structures depending on the position of groups A and B in space: they are geometric isomers .
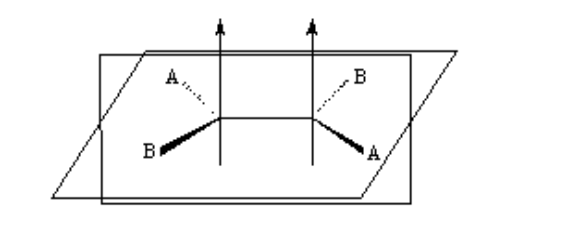
isomerism in alkenes
For geometric isomerism to exist, two conditions must be met:
1.- Impeded rotation (for example by a double bond)
2.- Two different groups (A and B) attached to both sides of the link
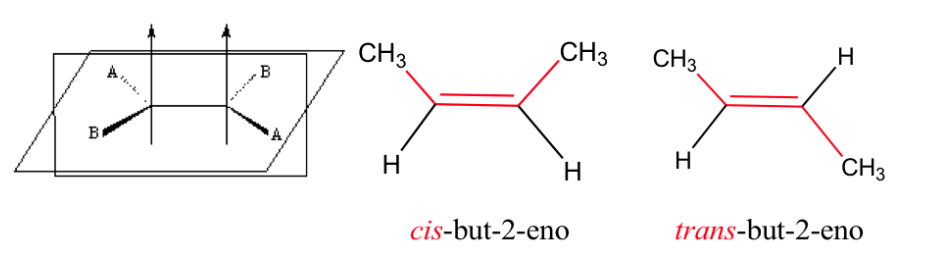
Effect of geometric isomerism on physical properties

Geometric isomer nomenclature
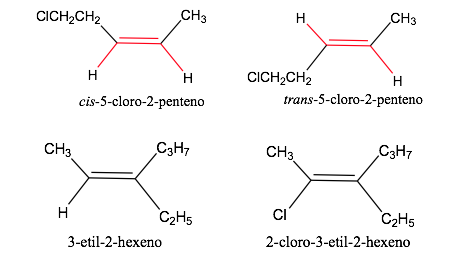 Sequence or priority rules
Sequence or priority rules
The rules that must be taken into account to establish the order of priority or preference of atoms or groups of atoms were established in 1956 by Cahn , Ingold and Prelog and modified several times to avoid ambiguities.
When the high-priority groups lie on opposite sides of the plane perpendicular to the molecule, the isomer is called E.
When they are on the same side of this plane, the isomer is called Z.
Example: but-2-ene:
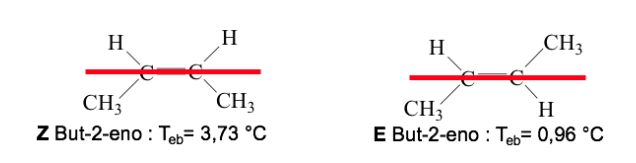
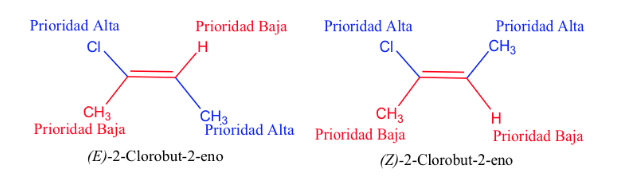
The priority of the substituents on the carbons of the double bond can be deduced from the following rules:
- Rule 1:
- If the atoms attached to the carbon atom under study are different, those with the higher atomic number have priority over those with the lower atomic number and if there are two isotopes, they are considered in decreasing order of atomic mass.
Ex: Br (35)> Cl (17)> O (8)> N (7)> C (6)> H (1)
D> H and 13 C> 12 C
Rule 2 :
When the atoms attached to the carbon atom are identical (and the first rule does not work), the sequence is followed, that is, the atoms attached to them are compared and, if necessary because they were also equal, the atoms attached to them are used. following, etc., taking into account that if the atoms are the same but in a different number, the substituent with more atoms of higher rank has priority.
Ex : -CH 2 -OH> -CH 3 because O> H
-CH 2 -Br> -CH 2 -OH because Br> OH
-CH 2 -CH 3> -CH 3 because C> H
Rule 3:
Double and triple bonds are treated as if they were single, doubling or tripling the atoms in the chain, respectively.
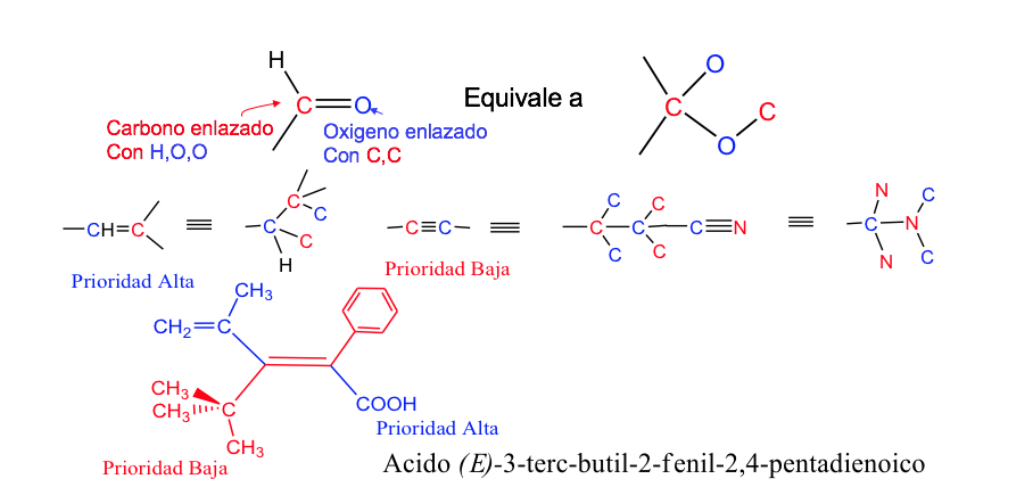
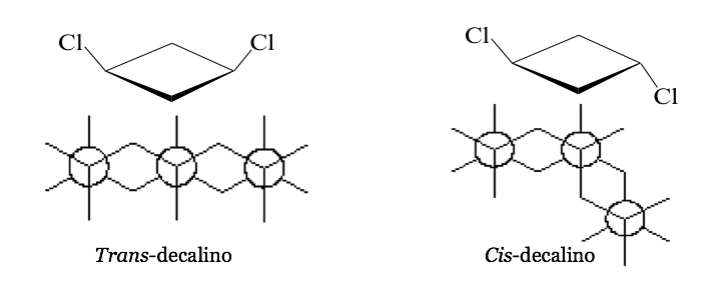
. Isomerism of cycles and complex systems.
In some symmetric (at least disubstituted) cyclic molecules, the atoms of the cycle define a plane. One substituent lies towards one face of this plane while the other may lie towards the same side or towards the opposite side.
cis- 1,3-Dichlorocyclobutane trans- 1,3-Dichlorocyclobutane
Projective representation (CRAM)
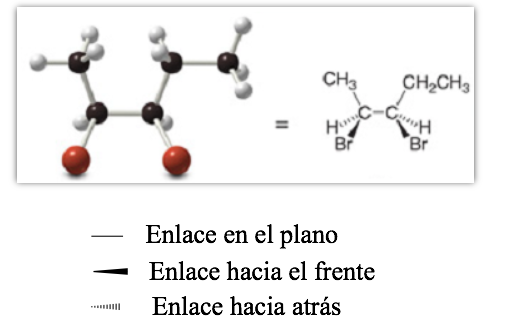
perspective representation
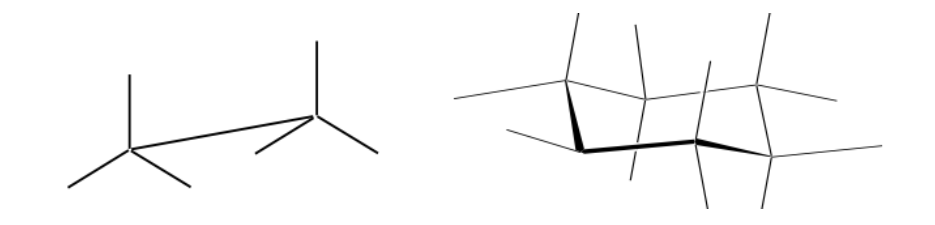
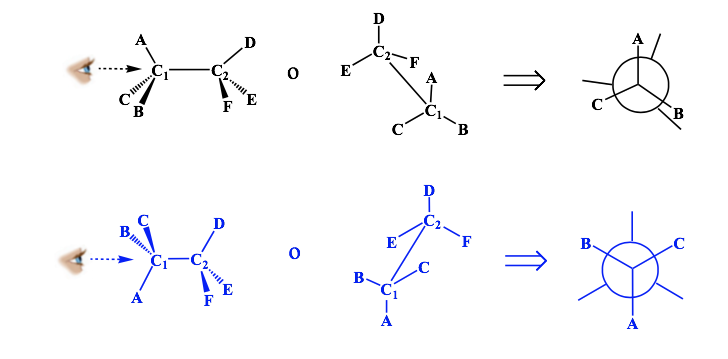
Newman representation
A Newman projection is a form of two-dimensional representation useful for visualizing conformations in a carbon-carbon single bond in an organic molecule.
Fisher representation.
The Fisher projection is a standard way of drawing tetrahedral carbon atoms and their substituents in two dimensions.
In this projection each tetrahedral carbon is represented as a cross in which the horizontal lines are directed out of the paper and the vertical ones inward.
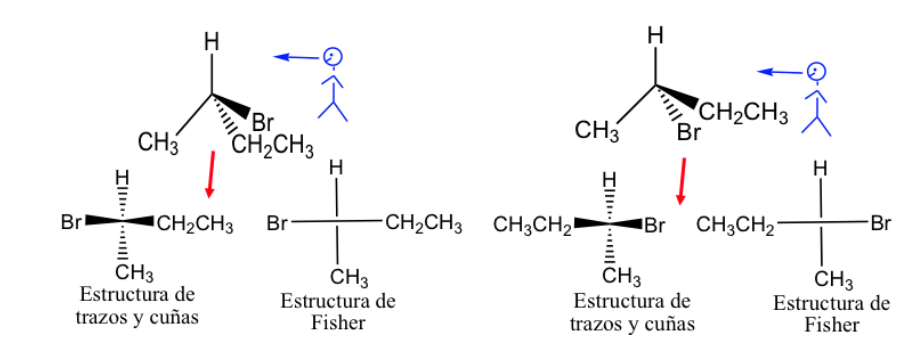
The Fisher representation can be done by marking the bonds that are in front of the plane with a thick line and those located behind with a dashed line, but generally the different links are presented with normal lines, although it is understood that the substituents represented to the right and left of the vertical line is above the plane of representation and those represented above and below are below that plane.
By general convention, the carbon chain is presented vertically, putting the most oxidized carbon at the top.
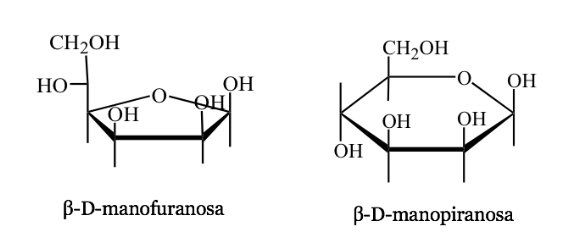
Harwoth Representation
- It is a perspective representation of the cyclic forms of sugar molecules with 5 or 6 atoms (furanoses, pyranoses).
- Eg:
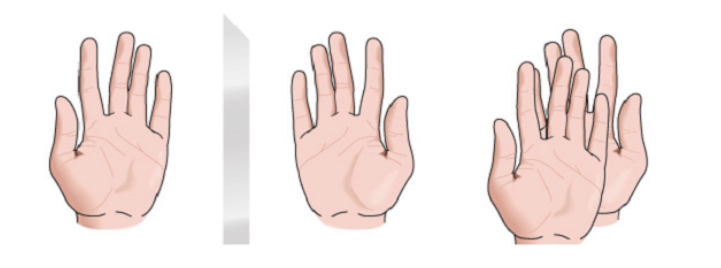
chirality
Any geometric figure, or any group of points, whose image in a plane mirror, ideally realized, cannot be made to coincide with itself, is called chiral. Some molecules are like hands. The left is the mirror image of the right but they are not superimposable and therefore not identical. They are called chiral .
There are other molecules similar to a pair of socks. The socks are mirror images of each other and are also superimposable.
A molecule or an object superimposable on its mirror image is called achiral .
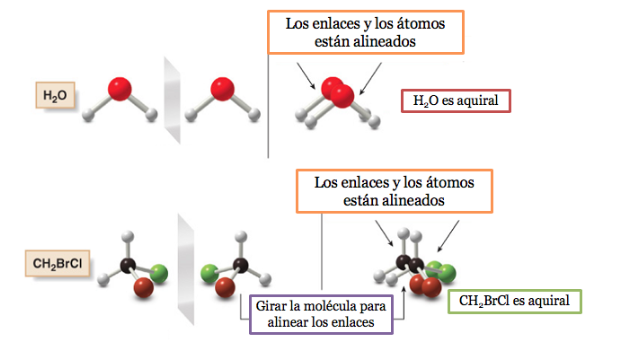
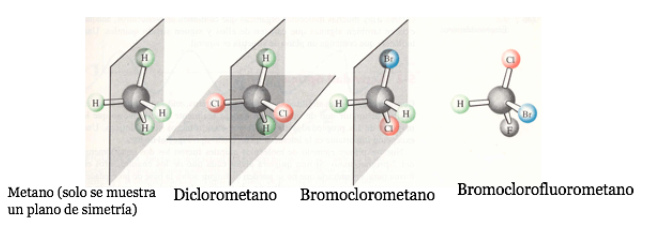
If a molecule has a plane of symmetry it is an achiral system.
A chiral molecule exists in two stereoisomeric forms called enantiomers . These are non-superimposable objects with their mirror images.
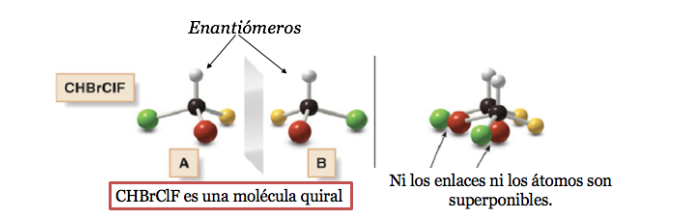
A carbon atom attached to 4 different substituents is called an asymmetric carbon. However, its existence is not a guarantee of chirality (as will be seen later). Also called stereogenic carbon or stereocenter.
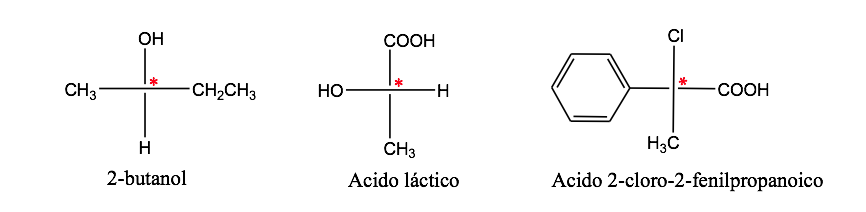
optical isomerism
The physical properties of two enantiomers are identical: they have the same boiling and melting points, the same solubility, the same density, the same refractive index, the same conductivity...etc.
The optical activity of the pairs of enantiomers is the characteristic property to differentiate them.
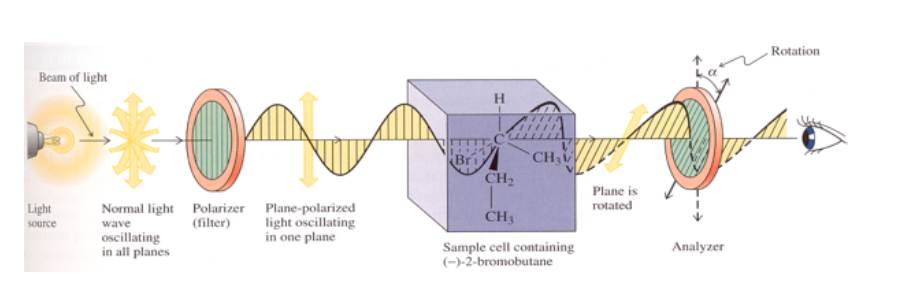
If the substance is not optically active, no change in the plane of vibration of the emitted polarized light is observed.
If the substance has optical activity, a rotation of to degrees of the plane of vibration of the emitted polarized light is observed.
If the rotation of the plane of light is to the right (in the same clockwise direction), the substance is dextrorotatory and the value α is assigned a positive sign.
If the rotation is to the left (counterclockwise) the substance is left-handed and α is assigned a negative sign.
The specific rotation of an optically active molecule is a characteristic physical constant of that molecule.
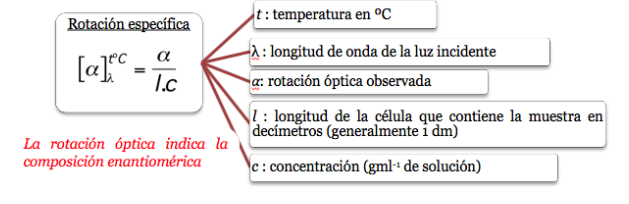
Specific rotation of some chiral compounds
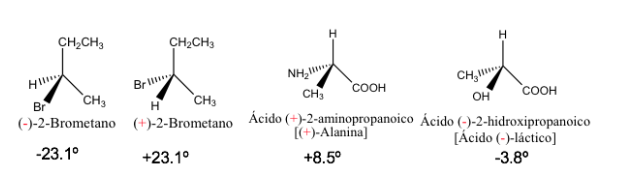
The pure enantiomers have the same value of specific rotation but with the opposite sign.
Therefore, the resulting optical rotation of a 1:1 mixture of enantiomers is zero, ie, it is optically inactive. This type of mixture is called a racemate or racemic mixture .
The naming of the absolute configuration of a stereogenic center is based on the same priority rules developed by Cahn, Ingold and Prelog .
These rules allow naming and describing the arrangement in space of substituents on a stereogenic center, regardless of the sign of the optical rotation of the molecule.
The lowest priority substituent is located as far away from the observer as possible.
If the step from 1 to 2 to 3 is done clockwise, the chiral center is R (rectus, Latin, right).
If the step 1 to 2 to 3 is done counterclockwise, the configuration of the chiral center is called S (sinister, Latin, left).
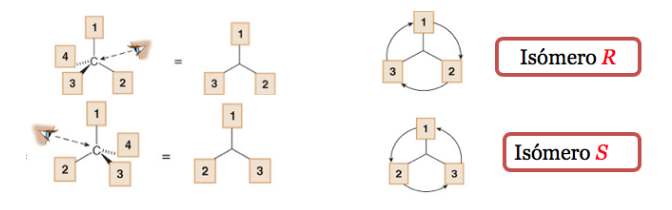
In systematic nomenclature, R or S are added in parentheses as a prefix to the name of the chiral compound.
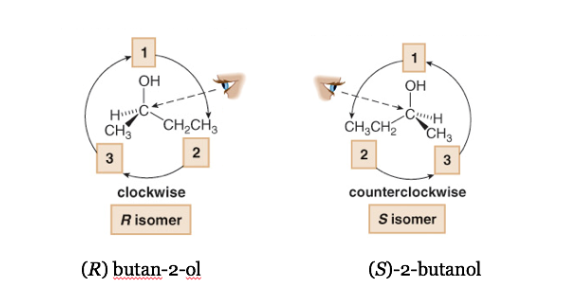
It is important to remember that the symbols R and S do not show any kind of correlation with the sign of α .
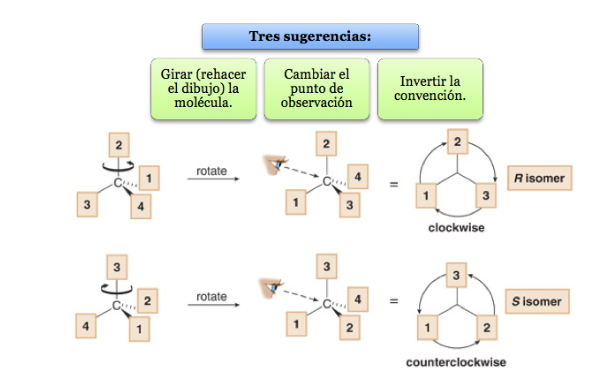
What to do when a molecule is not oriented so that the lowest priority group is far away?
A compound with n stereogenic centers has a maximum of 2 n stereoisomers.
Example:
A compound with two stereogenic centers has a maximum of 4 stereoisomers.
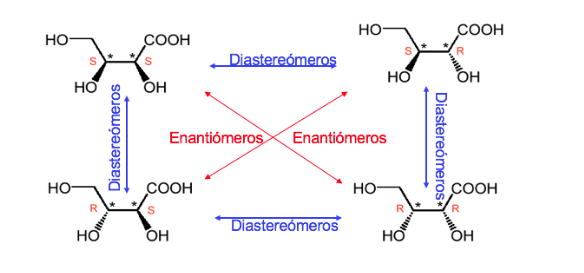
Any stereoisomer whose molecule is not chiral despite having stereogenic centers is called a meso form or compound.
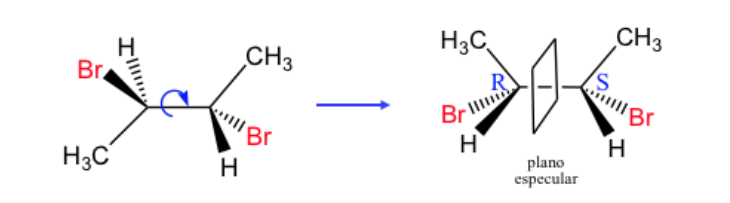
A compound with two asymmetric carbons is dominated like when the two carbons have the same absolute configuration and unlike in the opposite case.
erythro and threo compounds
When two carbons have at least two identical substituents, the designation threo and erythro can be used.
An erythro enantiomer pair is one in which identical groups can be placed in the eclipsed position.
There is no direct relationship between the R and S nomenclature and the Erythro/Treo nomenclature.
When two carbons have three identical substituents, the erythro form is meso since it presents a plane of symmetry.
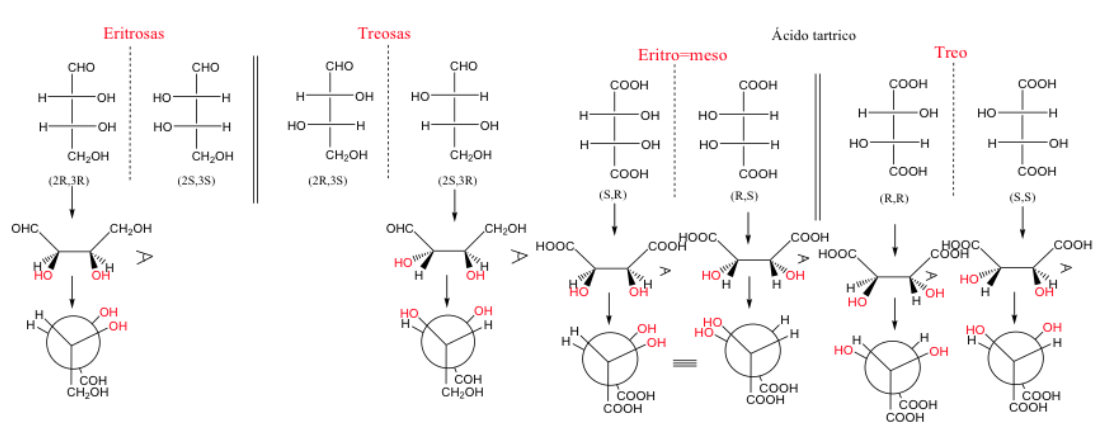
A molecule is called an erythro when, in its Fischer representation, the same or similar groups are on the same side.
A molecule is three if these groups are on opposite sides.
(generally used for sugars (osas))
It is a nomenclature that predates the R and S nomenclature.
A sugar is named D when, in the Fischer projection (with the most oxidized carbon located on top), the hydroxyl associated with the highest numbered asymmetric carbon is on the right.
Its enantiomer will be called L and will have the OH equivalent on the left.
Glucose and fructose in their natural forms exist as D.
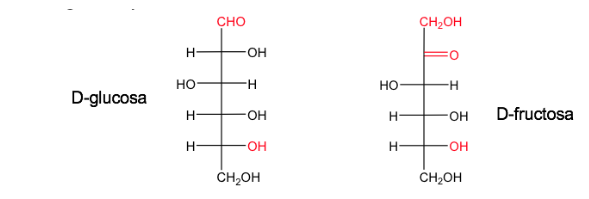
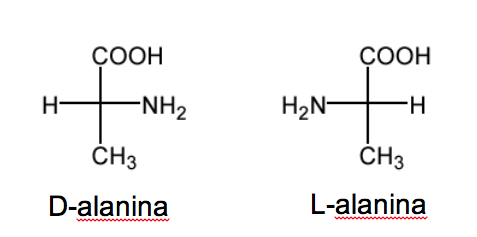
The D/L nomenclature is also used in the series of amino acids.
RCH( NH2 )COOH.
In sugars this nomenclature depends on the position of the hydroxyl. In this case it is the position of the amino group that defines the nomenclature. When in the Fisher projection (with the most oxidized carbon on top) the NH 2 group is on the right, the ethereoisomer is D and its enantiomer is L .
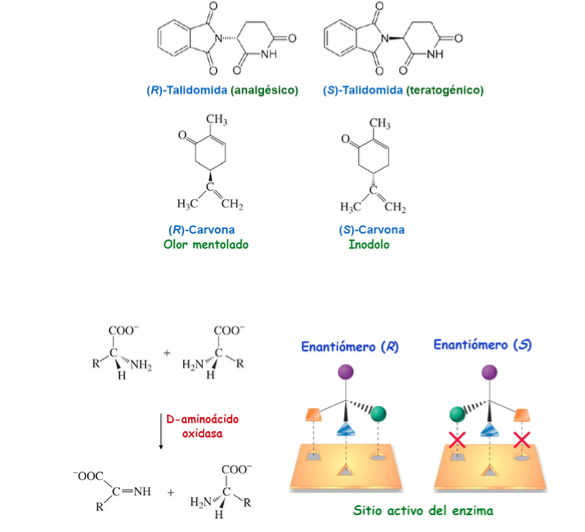
Importance of chirality
Sources to expand the search for knowledge:
1) Juaristi E. “Introduction to stereochemistry and conformational analysis”. CINVESTAV, Mexico, 1988 Juaristi E. . CINVESTAV, Mexico, 1994.
2) Neil SI “Physical Organic Chemistry” Longman, Milan, 1995.
3) March J., “Advanced Organic Chemistry” John Wiley & Sons, New York, 1992 4) Jones RAY “Physical and Mechanistic Organic Chemistry”, 2nd. Ed Cambridge University Press, Cambridge, 1984.
5) Woodward RB and Hoffmann R. “The conservation of orbital symmetry”, Academic Press, New York, 1979.
6) Carpenter BK “Determination of Organic Reaction Mechanisms”, John Wiley & Sons, New York, 1984 .