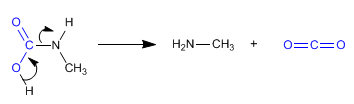
Amides are transformed into amines with one less carbon, when treated with bromine in basic solution.
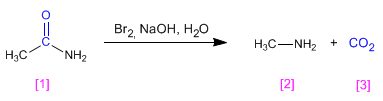
Acetamide [1] reacts with bromine in a basic medium, transforming into methylamine [2] with the loss of carbon dioxide.
The Hofmann rearrangement only occurs with amides having two hydrogens on the amino group (amines not substituted on the nitrogen).
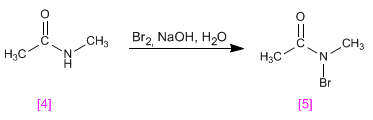
The amide, N-substituted, forms an N-bromo amide on reaction with bromine in a basic medium, but does not give the Hofmann rearrangement.
The Hofmann rearrangement mechanism occurs in the following stages:
Stage 1 . Amidate formation
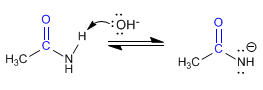
Stage 2. Reaction of amidate with bromine to form N-bromo amide.
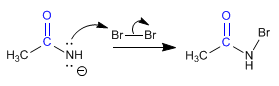
Stage 3. Formation of a new amidate, by deprotonation of nitrogen.
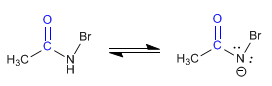
Stage 4. Bromine removal
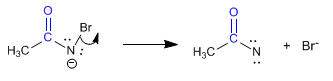
Stage 5. Transposition
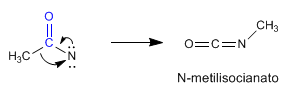
Stage 6. Attack of water on N-methylisocyanate to form carbamic acid
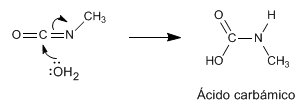
Stage 7. Decomposition of carbamic acid (unstable) to form the final amine and carbon dioxide.