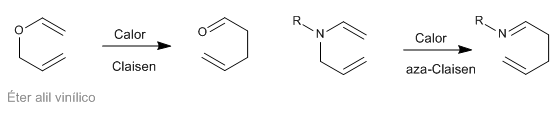
Sigmatropic [3,3] rearrangements of N-allyleneamines are known as aza-Claisen rearrangements. This reaction is analogous to the Claisen rearrangement of allyl vinyl ethers.
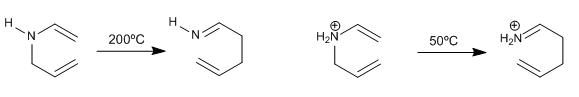
The reaction goes under milder conditions if the nitrogen is charged (ammonium salt)
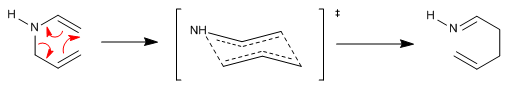
The aza-Claisen reaction is a concerted process that proceeds through a six-membered transition state that assumes a chair arrangement with the substituents arranged nearly equatorially.