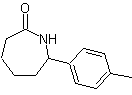
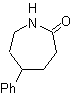
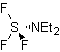The Beckmann transposition as a strategy in
The rearrangement of oximes in an acid medium, called the Beckman rearrangement, produces an amide or lactam if the starting ketone is linear or cyclic, respectively.
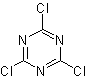
To improve the performance of this type of reaction, various catalysts and acid media have been studied. Thus, for example, new acid media used as catalysts, with the indicated purpose, are: TCT/DMF, DAST/CH 2 CL 2 , CF 3 SO 3 H, PCl 5 , HgCl 2 /MeCN and the ZnO.
TCT: trichlorotriazine | …… | DAST: diethylaminosulfide trifluoride |
The migratory fitness of the groups is the same as in the Baeyer-Villiger reaction. Propose a synthesis design for each of the following molecules:
MOb 62 | mob 63 | MOb 64 |
MOb 62 . Retrosynthetic analysis.
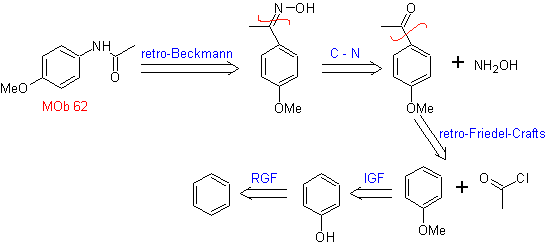
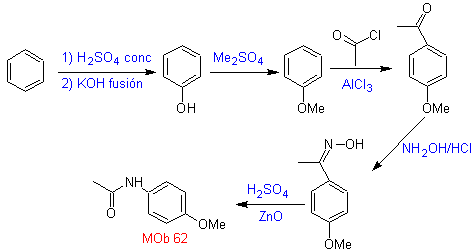
Synthesis. The reaction of the intermediate aromatic ketone with NH 2 OH and its subsequent treatment with an aqueous solution of sulfuric acid and ZnO, allows the formation of the aromatic amide MOb 62.
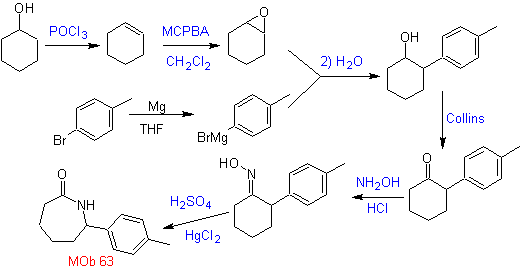
MOb 63. Retrosynthetic analysis.
The synthesis strategy
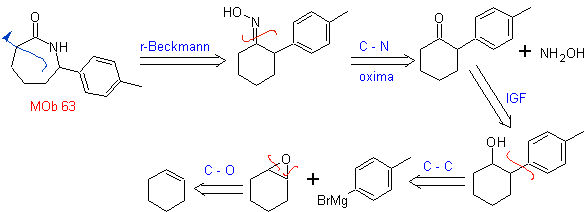
synthesis .
The synthesis of MOb 63, according to the assumed strategy, does not present any reaction of special care. The last reaction shows the proper use of the Beckmann rearrangement reaction for the formation of lactams.
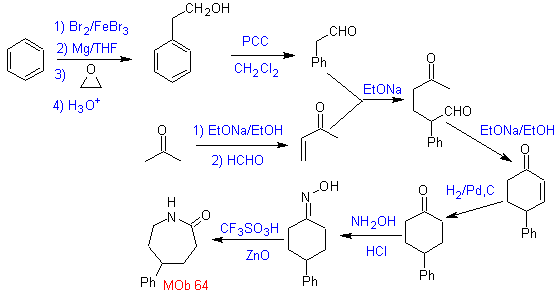
MOb 64. Retrosynthetic analysis. Beckmann's rearrangement strategy, for the phase of disconnection of
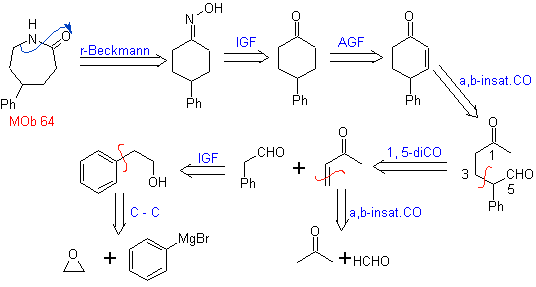
Synthesis. Benzene is a suitable starting material for the synthesis of