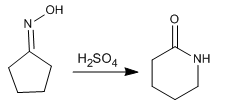
The Beckmann reaction produces the rearrangement of an oxime into an amide. This reaction is carried out in an acid medium.
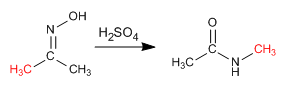
Mechanism:
Step 1. Protonation of the hydroxyl group
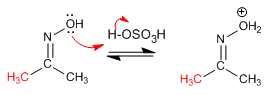
Stage 2. Migration of the substituent with simultaneous loss of water.
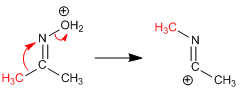
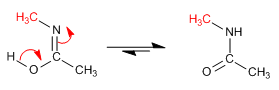
Stage 3. Attack of the water on the cation
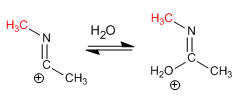
Stage 4. Formation of iminol by loss of a proton from water
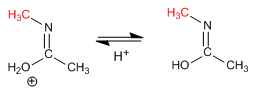
Stage 5. Iminol tautomerism
In the case of oximes from aldehydes (aldoximes), chain migration occurs, making it impossible to obtain unsubstituted amides.
The Beckmann rearrangement requires the ANTI arrangement of the migrating group with respect to the water molecule acting as the leaving group.
The Beckmann rearrangement produces lactams by reaction with cyclic oximes.