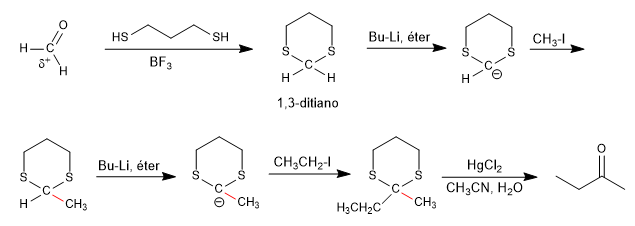
The 1,3-dithianes allow to change the polarity of the carbonyl carbon of the aldehydes by subtraction of the acid hydrogen, obtaining an organolithic capable of attacking a wide variety of electrophiles.
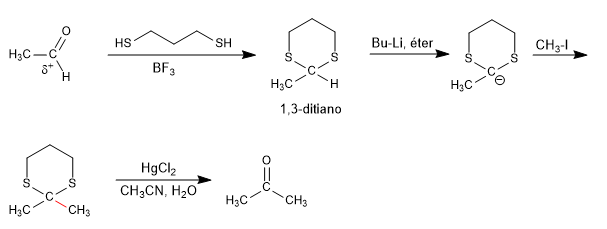
The initial carbonyl, with positive polarity on carbon, changes in the umpolung reaction to a carboanion. The sulphurs of 1,3-dithiane are vital in stabilizing the negative charge, and the reaction with the oxygenated equivalent is not viable.
Methanal is the most versatile aldehyde in these reactions as it has two hydrogens that can be replaced by electrophiles.