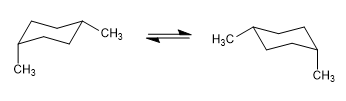
In the following model it can be seen that in the equatorial conformer the methyl group is far from the rest of the groups. On the contrary, in the axial conformer said methyl group is facing the axial hydrogens that are located in position 3 with respect to it. This spatial proximity causes a steric repulsion, called the 1,3-diaxial interaction.
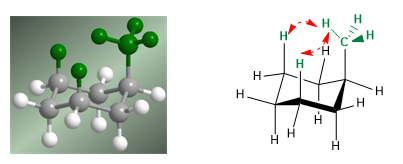
The 1,3-diaxial interaction causes a shift of the conformational equilibrium to the left. The conformation on the right has a high energy due to the repulsion between methyl and axial hydrogens.
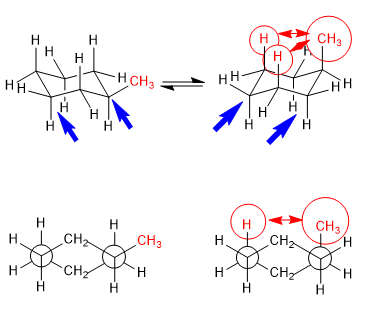
Equilibrium in trans-1,4-dimethylcyclohexane
The 1,3-diaxial interactions make the substituents tend to be located in equatorial positions. Thus, in trans-1,4-Dimethylcyclohexane the conformation with the two methyl groups in equatorial is more stable than the chair with axial methyls, this produces a displacement of the conformational equilibrium to the left.
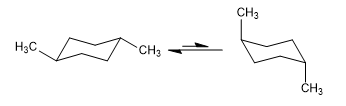
Equilibrium in cis-1,4-dimethylcyclohexane