Nitriles hydrolyze in acidic media, under heating, becoming carboxylic acids and ammonium salts. The hydrolysis of nitriles is an irreversible process.
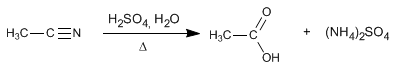
The reaction mechanism consists of the following stages:
Stage 1. Protonation of the nitrile
Stage 2. Nucleophilic attack of water
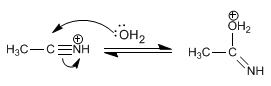
Stage 3. Deprotonation of water
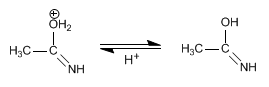
Stage 4. Tautomerism
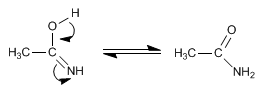
The amide hydrolyzes in the acid medium to carboxylic acid. The mechanism can be found in the section e amides.
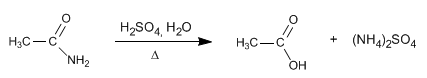
Due to the higher reactivity of the nitrile on the amide, working under mild conditions, the hydrolysis to the amide can be stopped.