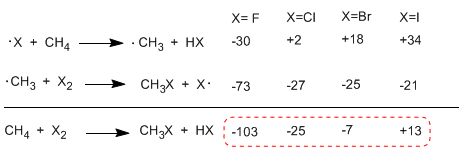When comparing the enthalpies of the propagation stages of the different halogens, important differences in the exchanged heats are observed. In the case of fluorine, both steps are exothermic (even the abstraction of hydrogen) with a global energy balance of -103 Kcal/mol. The activation energies of the transition states in this reaction are very low, making it the most reactive halogen.
At the other extreme of reactivity is iodine, whose reaction is endothermic and does not take place.
Order of reactivity F2>Cl2> Br2> I2
The following table shows the heat exchanged in the propagation stages for the halogenation of methane.
The reaction is strongly exothermic in the case of fluorine (halogen more reactive) while in the case of iodine it is endothermic at 13 Kcal/mol.