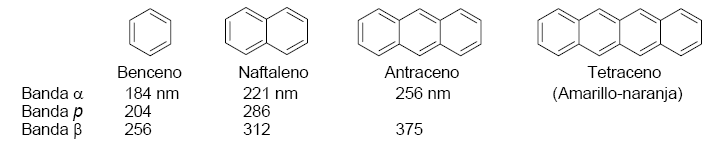
The absorption spectrum of benzene consists of three bands at 184, 204, and 256 nm, often called $\alpha$, p, and $\beta$. The $\alpha$, p bands are also known as primary and the $\beta$ secondary band. The secondary band is wide due to its vibrational structure.
Conjugation with other aromatic rings and with substituents possessing double bonds or lone pairs produces a bathochromic shift in the bands.