The reduction of alkanoyl halides can be stopped at the aldehyde using modified hydrides. From lithium aluminum hydride lithium tri(tert-butoxy)aluminum hydride can be prepared. This modified hydride allows reduction of acid halides to aldehydes.
Synthesis of the reductant: the reductant is obtained by reaction between lithium aluminum hydride and tert-butanol
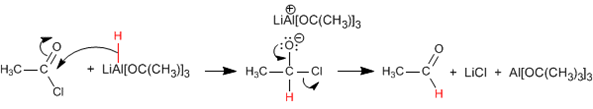
Acid Halide Reduction: Acid halides are reduced to aldehydes. The modified reducer at low temperature and using a single equivalent does not reduce aldehydes.
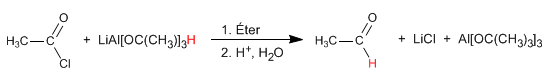
Mechanism: The reaction proceeds with the attack of the hydride generated by the reductant on the carbonyl carbon, to eliminate chloride in a second stage.