The Birch reduction uses sodium or lithium in solution as reagents, its mechanism is radical and reduces benzene to 1,4-cyclohexadiene.
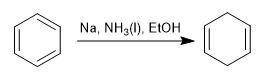
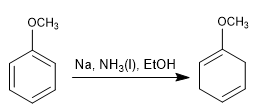
Birch with activating substituents
The double bonds of the final cyclohexadiene lie close to the substituents that activate the ring.
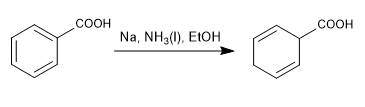
Birch with deactivating substituents
The double bonds of the final cyclohexadiene lie away from the substituents that deactivate the ring.
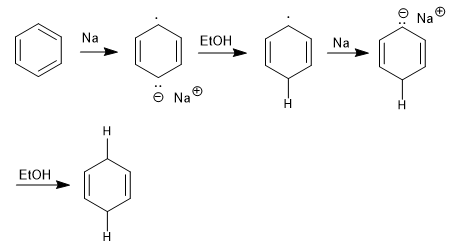
Birch mechanism
Birch is a reaction that occurs with the formation of an anion-radical.