Halogenated benzenes react with dilute soda under conditions of high pressure and temperature to form phenols. This reaction does not require deactivating groups in the ortho/para position and follows a different mechanism than aromatic nucleophilic substitution by addition-elimination.
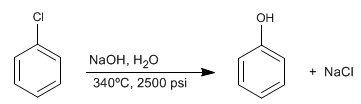
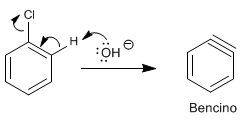
This reaction was discovered in 1928 by chemists at the Dow Chemical Company. The mechanism consists in the elimination of HCl with formation of an unstable intermediate called benzine, which is attacked by the hydroxide ions of the medium, to form phenol.
Stage 1. HCl removal
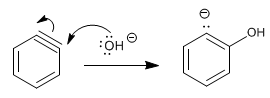
Stage 2. Addition of the hydroxide ion to the benzine
Stage 3. Protonation
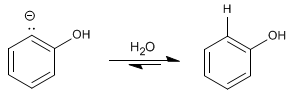
![]() The mechanism of this reaction is called elimination-addition aromatic nucleophilic substitution.
The mechanism of this reaction is called elimination-addition aromatic nucleophilic substitution.
When there are substituents in benzene, it produces mixtures, due to the attack of the nucleophile on the two carbons of the triple bond.