The carbon attached directly to benzene is known as the benzylic position. In this position, highly stable carbocations, carbanions and radicals are formed due to the possibility of delocalizing the charge on the aromatic ring.
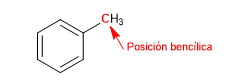
SN1 in benzylic positions
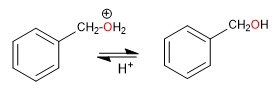
Primary haloalkanes on benzylic positions give SN1 since the primary carbocation delocalizes by resonance within benzene.
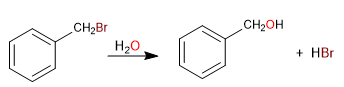
Mechanism:
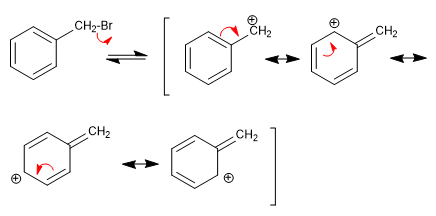
Stage 1. Loss of the leaving group, with formation of a benzylic cation.
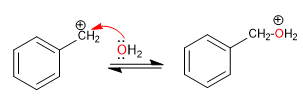
Stage 2. Nucleophilic attack of water on the benzyl cation
Stage 3. Deprotonation
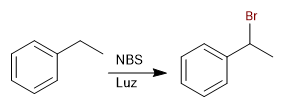
Halogenation of the benzylic position.
The benzylic positions are selectively halogenated using NBS.
Acidity of benzylic hydrogens
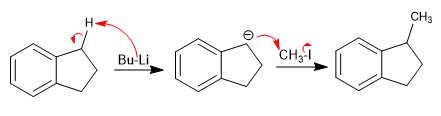
Hydrogens located in benzylic positions can be subtracted using strong bases such as LDA and organometallics.
