Draw the products of acid- and base-catalyzed bromination of acetylcyclopentane. Indicate the major product. Reason your answer.
SOLUTION:
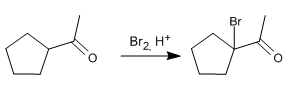
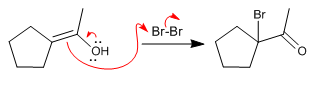
Mechanism:
Stage 1. Formation of the enol

Stage 2. Attack of the enol on the bromine molecule
In basic media, methyl ketone produces the bromoform reaction.
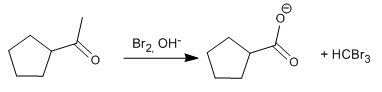
The mechanism of this last reaction can be found at the address: http://www.quimicaorganica.org/enolatos-y-enoles/247-reaccion-del-haloformo-iodoformo.html