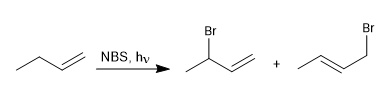
Halogens can add to alkenes giving vicinal dihaloalkanes, but when this reaction is carried out at low halogen concentrations, radical mechanisms are favored. A widely used reagent in allylic brominations is NBS (N-bromosuccinimide).
Halogenation mechanism
This process begins with the dissociation of the NBS in the presence of light. The bromo radicals cleave the allylic hydrogen, forming a resonance-stabilized radical that reacts with the NBS to give a mixture of products.
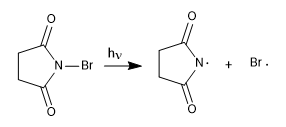
Stage 1. Initiation
Stage 2. Propagation
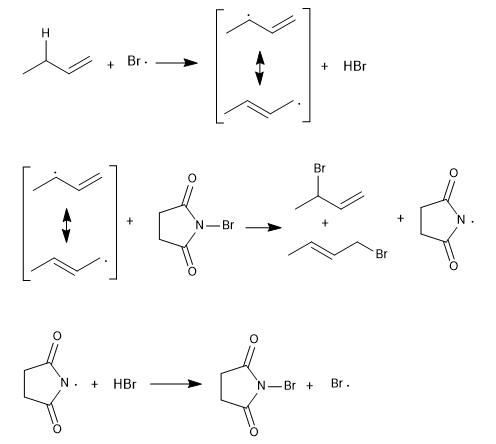
Stage 3. Termination.
When the reactants run out, the radicals begin to unite with each other, generating traces of products that are not considered in the overall reaction.