In propane there are two types of non-equivalent hydrogens that can be replaced by halogen, obtaining 1-chloropropane and 2-chloropropane.
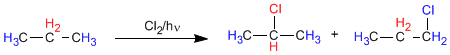
The proportion in which these products are obtained depends on two factors: the number of hydrogens to be replaced by halogen and the stability of the radical formed.
Number of hydrogens
Since propane has 6 primary and 2 secondary hydrogens, the ratio of products should be 3 to 1.
Radical stability
However, the secondary CH bonds are weaker than the primary ones and the removal of a secondary hydrogen by the chlorine radical is faster than that of a primary one (approximately 4 times faster). These differences in the speed of subtraction are due to the greater stability of the secondary radical over the primary one. These data allow us to calculate the proportion in which both products are obtained.
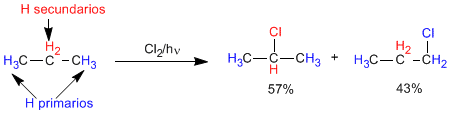
relative speeds
F(1 : 1.2 : 1.4), Cl(1 : 4 : 5), Br(1 : 250 : 6300). The first number indicates the speed with which a primary hydrogen is replaced by the corresponding halogen. The second value tells us the speed of substitution of a secondary hydrogen by halogen. The third value applies to tertiary hydrogens.
Selectivity and reactivity
The more reactive the halogen is, the less it distinguishes between primary, secondary, or tertiary hydrogens. Fluorine halogenates all three types of hydrogens almost equally and is said to be poorly selective. On the contrary, bromine is very unreactive and looks for the easiest hydrogens to remove (the tertiary ones) and replaces them 6300 times faster than the primary ones. It is said of him that he is very selective.
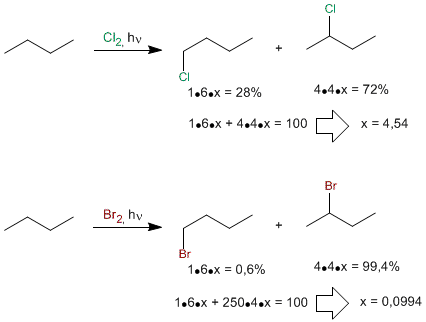
The low reactivity of bromine makes it have a high selectivity, halogenating in a very majority the secondary position compared to the primary one. As can be seen, chlorine is much less selective.